Epstein-Barr virus protein kinase BGLF4 is a virion tegument protein that dissociates from virions in a phosphorylation-dependent process and phosphorylates the viral immediate-early protein BZLF1
- PMID: 16698993
- PMCID: PMC1472150
- DOI: 10.1128/JVI.02674-05
Epstein-Barr virus protein kinase BGLF4 is a virion tegument protein that dissociates from virions in a phosphorylation-dependent process and phosphorylates the viral immediate-early protein BZLF1
Abstract
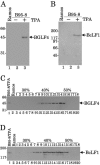
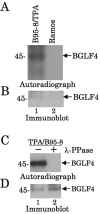
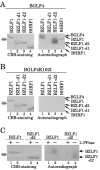
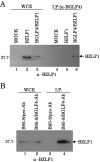
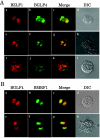
Epstein-Barr virus (EBV) BGLF4 is a viral protein kinase that is expressed in the lytic phase of infection and is packaged in virions. We report here that BGLF4 is a tegument protein that dissociates from the virion in a phosphorylation-dependent process. We also present evidence that BGLF4 interacts with and phosphorylates BZLF1, a key viral regulator of lytic infection. These conclusions are based on the following observations. (i) In in vitro tegument release assays, a significant fraction of BGLF4 was released from virions in the presence of physiological NaCl concentrations. (ii) Addition of physiological concentrations of ATP and MgCl(2) to virions enhanced BGLF4 release, but phosphatase treatment of virions significantly reduced BGLF4 release. (iii) A recombinant protein containing a domain of BZLF1 was specifically phosphorylated by purified recombinant BGLF4 in vitro, and BGLF4 altered BZLF1 posttranslational modification in vivo. (iv) BZLF1 was specifically coimmunoprecipitated with BGLF4 in 12-O-tetradecanoylphorbol-13-acetate-treated B95-8 cells and in COS-1 cells transiently expressing both of these viral proteins. (v) BGLF4 and BZLF1 were colocalized in intranuclear globular structures, resembling the viral replication compartment, in Akata cells treated with anti-human immunoglobulin G. Our results suggest that BGLF4 functions not only in lytically infected cells by phosphorylating viral and cellular targets but also immediately after viral penetration like other herpesvirus tegument proteins.
Figures


References
-
- Chee, M. S., G. L. Lawrence, and B. G. Barrell. 1989. Alpha-, beta- and gammaherpesviruses encode a putative phosphotransferase. J. Gen. Virol. 70:1151-1160. - PubMed
-
- Cohen, J. I., and S. E. Straus. 2001. Varicella-zoster virus and its replication, p. 2707-2730. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott-Williams & Wilkins, Philadelphia, Pa.
-
- Edelman, A. M., D. K. Blumenthal, and E. G. Krebs. 1987. Protein serine/threonine kinases. Annu. Rev. Biochem. 56:567-613. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

