Ebola virus VP35 protein binds double-stranded RNA and inhibits alpha/beta interferon production induced by RIG-I signaling
- PMID: 16698997
- PMCID: PMC1472134
- DOI: 10.1128/JVI.02199-05
Ebola virus VP35 protein binds double-stranded RNA and inhibits alpha/beta interferon production induced by RIG-I signaling
Abstract
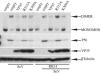
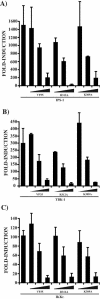
The Ebola virus (EBOV) VP35 protein blocks the virus-induced phosphorylation and activation of interferon regulatory factor 3 (IRF-3), a transcription factor critical for the induction of alpha/beta interferon (IFN-alpha/beta) expression. However, the mechanism(s) by which this blockage occurs remains incompletely defined. We now provide evidence that VP35 possesses double-stranded RNA (dsRNA)-binding activity. Specifically, VP35 bound to poly(rI) . poly(rC)-coated Sepharose beads but not control beads. In contrast, two VP35 point mutants, R312A and K309A, were found to be greatly impaired in their dsRNA-binding activity. Competition assays showed that VP35 interacted specifically with poly(rI) . poly(rC), poly(rA) . poly(rU), or in vitro-transcribed dsRNAs derived from EBOV sequences, and not with single-stranded RNAs (ssRNAs) or double-stranded DNA. We then screened wild-type and mutant VP35s for their ability to target different components of the signaling pathways that activate IRF-3. These experiments indicate that VP35 blocks activation of IRF-3 induced by overexpression of RIG-I, a cellular helicase recently implicated in the activation of IRF-3 by either virus or dsRNA. Interestingly, the VP35 mutants impaired for dsRNA binding have a decreased but measurable IFN antagonist activity in these assays. Additionally, wild-type and dsRNA-binding-mutant VP35s were found to have equivalent abilities to inhibit activation of the IFN-beta promoter induced by overexpression of IPS-1, a recently identified signaling molecule downstream of RIG-I, or by overexpression of the IRF-3 kinases IKKepsilon and TBK-1. These data support the hypothesis that dsRNA binding may contribute to VP35 IFN antagonist function. However, additional mechanisms of inhibition, at a point proximal to the IRF-3 kinases, most likely also exist.
Figures



References
-
- Basler, C. F., and A. Garcia-Sastre. 2002. Viruses and the type I interferon antiviral system: induction and evasion. Int. Rev. Immunol. 21:305-337. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

