Evidence for potent autologous neutralizing antibody titers and compact envelopes in early infection with subtype C human immunodeficiency virus type 1
- PMID: 16699001
- PMCID: PMC1472127
- DOI: 10.1128/JVI.00201-06
Evidence for potent autologous neutralizing antibody titers and compact envelopes in early infection with subtype C human immunodeficiency virus type 1
Erratum in
- J Virol. 2007 Nov;81(22):12716
Abstract
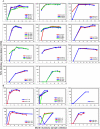
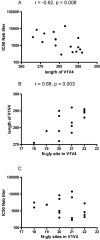
Information about neutralizing antibody responses in subtype C-infected individuals is limited, even though this viral subtype causes the majority of AIDS cases worldwide. Here we compared the course and magnitude of the autologous neutralizing antibody (NAb) response against viral envelope (Env) glycoproteins present during acute and early infection with subtypes B and C human immunodeficiency virus type 1 (HIV-1). NAb responses were evaluated in 6 subtype B-infected and 11 subtype C-infected subjects over a mean evaluation period of 25 months using a pseudovirus reporter gene assay. All subjects in the C cohort were infected through heterosexual contact, while five of the six subjects in the B cohort were infected via male-to-male contact. The kinetics and magnitude of the NAb responses varied among subjects in the B and C cohorts; however, the median 50% inhibitory concentration (IC(50) titer) reached by antibody in the plasma of subtype C-infected subjects, overall, was 3.5-fold higher than in the subtype B-infected subjects (P = 0.06). The higher titers of NAbs in the C cohort were associated with viruses having significantly shorter amino acid length (P = 0.002) in the V1 to V4 region of the surface Env glycoprotein, gp120, compared to the B cohort. Despite the potency of the autologous subtype C NAb response, it was not directed against cross-neutralizing epitopes. These data demonstrate that subtype C Envs elicit a potent yet restricted NAb response early in infection that frequently reaches IC(50) titers in excess of 1:1,000 and suggest that clade-specific differences may exist in Env immunogenicity or susceptibility to neutralization.
Figures

References
-
- Albert, J., B. Abrahamsson, K. Nagy, E. Aurelius, H. Gaines, G. Nystrom, and E. M. Fenyo. 1990. Rapid development of isolate-specific neutralizing antibodies after primary HIV-1 infection and consequent emergence of virus variants which resist neutralization by autologous sera. AIDS 4:107-112. - PubMed
-
- Arendrup, M., C. Nielsen, J. E. Hansen, C. Pedersen, L. Mathiesen, and J. O. Nielsen. 1992. Autologous HIV-1 neutralizing antibodies: emergence of neutralization-resistant escape virus and subsequent development of escape virus neutralizing antibodies. J. Acquir. Immune Defic. Syndr. 5:303-307. - PubMed
-
- Binley, J. M., T. Wrin, B. Korber, M. B. Zwick, M. Wang, C. Chappey, G. Stiegler, R. Kunert, S. Zolla-Pazner, H. Katinger, C. J. Petropoulos, and D. R. Burton. 2004. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 78:13232-13252. - PMC - PubMed
-
- Bradney, A. P., S. Scheer, J. M. Crawford, S. P. Buchbinder, and D. C. Montefiori. 1999. Neutralization escape in human immunodeficiency virus type 1-infected long-term nonprogressors. J. Infect. Dis. 179:1264-1267. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

