Extraintestinal spread and replication of a homologous EC rotavirus strain and a heterologous rhesus rotavirus in BALB/c mice
- PMID: 16699002
- PMCID: PMC1472171
- DOI: 10.1128/JVI.02664-05
Extraintestinal spread and replication of a homologous EC rotavirus strain and a heterologous rhesus rotavirus in BALB/c mice
Abstract
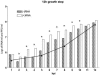
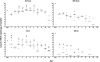
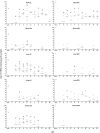
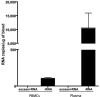
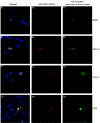
Although rotavirus infection has generally been felt to be restricted to the gastrointestinal tract, over the last two decades there have been sporadic reports of children with acute or fatal cases of rotavirus gastroenteritis testing positive for rotavirus antigen and/or nucleic acid in various extraintestinal locations such as serum, liver, kidney, bladder, testes, nasal secretions, cerebrospinal fluid, and the central nervous system. Recently, studies in animals and people have demonstrated that rotavirus antigenemia is a common event during natural infection. In this study, we extend these observations and compare the intestinal and extraintestinal spread of wild-type homologous murine rotavirus EC and a heterologous strain, rhesus rotavirus (RRV), in newborn mice. A strand-specific quantitative reverse transcription-PCR (ssQRT-PCR) assay was used to quantify the ability of different rotavirus strains to spread and replicate extraintestinally. Both strain EC and RRV were detected extraintestinally in the mesenteric lymph nodes (MLN), livers, lungs, blood, and kidneys. Extraintestinal replication, as measured by ssQRT-PCR, was most prominent in the MLN and occurred to a lesser degree in the livers, kidneys, and lungs. In the MLN, strain EC and RRV had similar (P < 0.05) RNA copy numbers, although EC was present at a 10,000-fold excess over RRV in the small intestine. Rotavirus nonstructural protein 4 (NSP4) and/or assembled triple-layered particles, indicated by immunostaining with the VP7 conformation-dependent monoclonal antibody 159, were detected in the MLN, lungs, and livers of EC- and RRV-inoculated mice, confirming the ssQRT-PCR findings. Infectious RRV was detected in the MLN in quantities exceeding the amount present in the small intestines or blood. The cells in the MLN that supported rotavirus replication included dendritic cells and potentially B cells and macrophages. These data indicate that extraintestinal spread and replication occurs commonly during homologous and some heterologous rotaviral infections; that the substantial host range restrictions for rhesus rotavirus, a heterologous strain present in the intestine, are not necessarily apparent at systemic sites; that the level and location of extraintestinal replication varies between strains; that replication can occur in several leukocytes subsets; and that extraintestinal replication is likely a part of the normal pathogenic sequence of homologous rotavirus infection.
Figures

References
-
- Blutt, S. E., C. D. Kirkwood, V. Parreno, K. L. Warfield, M. Ciarlet, M. K. Estes, K. Bok, R. F. Bishop, and M. E. Conner. 2003. Rotavirus antigenaemia and viraemia: a common event? Lancet 362:1445-1449. - PubMed
-
- Boshuizen, J. A., J. H. Reimerink, A. M. Korteland-van Male, V. J. van Ham, J. Bouma, G. J. Gerwig, M. P. Koopmans, H. A. Buller, J. Dekker, and A. W. Einerhand. 2005. Homeostasis and function of goblet cells during rotavirus infection in mice. Virology 337:210-221. - PubMed
-
- Brown, K. A., and P. A. Offit. 1998. Rotavirus-specific proteins are detected in murine macrophages in both intestinal and extraintestinal lymphoid tissues. Microb. Pathog. 24:327-331. - PubMed
-
- Burns, J. W., A. A. Krishnaney, P. T. Vo, R. V. Rouse, L. J. Anderson, and H. B. Greenberg. 1995. Analyses of homologous rotavirus infection in the mouse model. Virology 207:143-153. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

