Gag regulates association of human immunodeficiency virus type 1 envelope with detergent-resistant membranes
- PMID: 16699009
- PMCID: PMC1472128
- DOI: 10.1128/JVI.01469-05
Gag regulates association of human immunodeficiency virus type 1 envelope with detergent-resistant membranes
Abstract
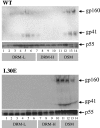
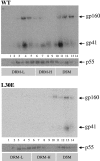
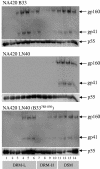
Assembly of the human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein on budding virus particles is important for efficient infection of target cells. In infected cells, lipid rafts have been proposed to form platforms for virus assembly and budding. Gag precursors partly associate with detergent-resistant membranes (DRMs) that are believed to represent lipid rafts. The cytoplasmic domain of the envelope gp41 usually carries palmitate groups that were also reported to confer DRM association. Gag precursors confer budding and carry envelope glycoproteins onto virions via specific Gag-envelope interactions. Thus, specific mutations in both the matrix domain of the Gag precursor and gp41 cytoplasmic domain abrogate envelope incorporation onto virions. Here, we show that HIV-1 envelope association with DRMs is directly influenced by its interaction with Gag. Thus, in the absence of Gag, envelope fails to associate with DRMs. A mutation in the p17 matrix (L30E) domain in Gag (Gag L30E) that abrogates envelope incorporation onto virions also eliminated envelope association with DRMs in 293T cells and in the T-cell line, MOLT 4. These observations are consistent with a requirement for an Env-Gag interaction for raft association and subsequent assembly onto virions. In addition to this observation, we found that mutations in the gp41 cytoplasmic domain that abrogated envelope incorporation onto virions and impaired infectivity of cell-free virus also eliminated envelope association with DRMs. On the basis of these observations, we propose that Gag-envelope interaction is essential for efficient envelope association with DRMs, which in turn is essential for envelope budding and assembly onto virus particles.
Figures




References
-
- Benn, S., R. Rutledge, T. Folks, J. Gold, L. Baker, J. McCormick, P. Feorino, P. Piot, T. Quinn, and M. Martin. 1985. Genomic heterogeneity of AIDS retroviral isolates from North America and Zaire. Science 230:949-951. - PubMed
-
- Brown, D. A., and E. London. 1998. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14:111-136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

