A short isoform of human cytomegalovirus US3 functions as a dominant negative inhibitor of the full-length form
- PMID: 16699020
- PMCID: PMC1472136
- DOI: 10.1128/JVI.02397-05
A short isoform of human cytomegalovirus US3 functions as a dominant negative inhibitor of the full-length form
Abstract
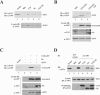
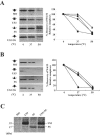
Human cytomegalovirus encodes four unique short (US) region proteins, each of which is independently sufficient for causing the down-regulation of major histocompatibility complex (MHC) class I molecules on the cell surface. This down-regulation enables infected cells to evade recognition by cytotoxic T lymphocytes (CTLs) but makes them vulnerable to lysis by natural killer (NK) cells, which lyse those cells that lack MHC class I molecules. The 22-kDa US3 glycoprotein is able to down-regulate the surface expression of MHC class I molecules by dual mechanisms: direct endoplasmic reticulum retention by physical association and/or tapasin inhibition. The alternative splicing of the US3 gene generates two additional products, including 17-kDa and 3.5-kDa truncated isoforms; however, the functional significance of these isoforms during viral infection is unknown. Here, we describe a novel mode of self-regulation of US3 function that uses the endogenously produced truncated isoform. The truncated isoform itself neither binds to MHC class I molecules nor prevents the full-length US3 from interacting with MHC class I molecules. Instead, the truncated isoform associates with tapasin and competes with full-length US3 for binding to tapasin; thus, it suppresses the action of US3 that causes the disruption of the function of tapasin. Our results indicate that the truncated isoform of the US3 locus acts as a dominant negative regulator of full-length US3 activity. These data reflect the manner in which the virus has developed temporal survival strategies during viral infection against immune surveillance involving both CTLs and NK cells.
Figures



References
-
- Ahn, K., A. Gruhler, B. Galocha, T. R. Jones, E. J. Wiertz, H. L. Ploegh, P. A. Peterson, Y. Yang, and K. Fruh. 1997. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity 6:613-621. - PubMed
-
- Andersson, M., S. Paabo, T. Nilsson, and P. A. Peterson. 1985. Impaired intracellular transport of class I MHC antigens as a possible means for adenoviruses to evade immune surveillance. Cell 43:215-222. - PubMed
-
- Beck, S., and B. G. Barrell. 1988. Human cytomegalovirus encodes a glycoprotein homologous to MHC class-I antigens. Nature 331:269-272. - PubMed
-
- Bennett, E. M., J. R. Bennink, J. W. Yewdell, and F. M. Brodsky. 1999. Cutting edge: adenovirus E19 has two mechanisms for affecting class I MHC expression. J. Immunol. 162:5049-5052. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

