A herpes simplex virus recombinant that exhibits a single-chain antibody to HER2/neu enters cells through the mammary tumor receptor, independently of the gD receptors
- PMID: 16699034
- PMCID: PMC1472129
- DOI: 10.1128/JVI.02725-05
A herpes simplex virus recombinant that exhibits a single-chain antibody to HER2/neu enters cells through the mammary tumor receptor, independently of the gD receptors
Abstract
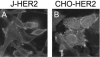
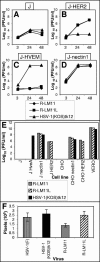
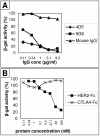
The human epidermal growth factor receptor 2/neuregulin (HER2/neu) receptor is overexpressed in highly malignant mammary and ovarian tumors and correlates with a poor prognosis. It is a target for therapy; humanized monoclonal antibodies to HER2 have led to increased survival of patients with HER2/neu-positive breast cancer. As a first step in the design of an oncolytic herpes simplex virus able to selectively infect HER2/neu-positive cells, we constructed two recombinants, R-LM11 and R-LM11L, that carry a single-chain antibody (scFv) against HER2 inserted at residue 24 of gD. The inserts were 247 or 256 amino acids long, and the size of the gD ectodomain was almost doubled by the insertion. We report the following. R-LM11 and R-LM11L infected derivatives of receptor-negative J or CHO cells that expressed HER2/neu as the sole receptor. Entry was dependent on HER2/neu, since it was inhibited in a dose-dependent manner by monoclonal antibodies to HER2/neu and by a soluble form of the receptor. The scFv insertion in gD disrupted the ability of the virus to enter cells through HVEM but maintained the ability to enter through nectin1. This report provides proof of principle that gD can tolerate fusion to a heterologous protein almost as large as the gD ectodomain itself without loss of profusion activity. Because the number of scFv's to a variety of receptors is continually increasing, this report makes possible the specific targeting of herpes simplex virus to a large collection of cell surface molecules for both oncolytic activity and visualization of tumor cells.
Figures




References
-
- Advani, S. J., R. R. Weichselbaum, R. J. Whitley, and B. Roizman. 2002. Friendly fire: redirecting herpes simplex virus-1 for therapeutic applications. Clin. Microbiol. Infect. 8:551-563. - PubMed
-
- Andreansky, S., L. Soroceanu, E. R. Flotte, J. Chou, J. M. Markert, G. Y. Gillespie, B. Roizman, and R. J. Whitley. 1997. Evaluation of genetically engineered herpes simplex viruses as oncolytic agents for human malignant brain tumors. Cancer Res. 57:1502-1509. - PubMed
-
- Bergman, I., P. Whitaker-Dowling, Y. Gao, and J. A. Griffin. 2004. Preferential targeting of vesicular stomatitis virus to breast cancer cells. Virology 330:24-33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

