Two surface-exposed elements of the B30.2/SPRY domain as potency determinants of N-tropic murine leukemia virus restriction by human TRIM5alpha
- PMID: 16699044
- PMCID: PMC1472168
- DOI: 10.1128/JVI.00219-06
Two surface-exposed elements of the B30.2/SPRY domain as potency determinants of N-tropic murine leukemia virus restriction by human TRIM5alpha
Abstract
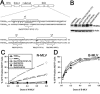
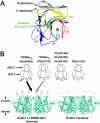
Human TRIM5alpha (TRIM5alpha(hu)) potently restricts N-tropic (N-MLV), but not B-tropic, murine leukemia virus in a manner dependent upon residue 110 of the viral capsid. Rhesus monkey TRIM5alpha (TRIM5alpha(rh)) inhibits N-MLV only weakly. The study of human-monkey TRIM5alpha chimerae revealed that both the v1 and v3 variable regions of the B30.2/SPRY domain contain potency determinants for N-MLV restriction. These variable regions are predicted to be surface-exposed elements on one face of the B30.2 domain. Acidic residues in v3 complement basic residue 110 of the N-MLV capsid. The results support recognition of the retroviral capsid by the TRIM5alpha B30.2 domain.
Figures


References
-
- Bieniasz, P. D. 2003. Restriction factors: a defense against retroviral infection. Trends Microbiol. 11:286-291. - PubMed
-
- Goff, S. P. 2004. Genetic control of retrovirus susceptibility in mammalian cells. Annu. Rev. Genet. 38:61-85. - PubMed
-
- Grutter, C., C. Briand, G. Capitani, P. R. Mittl, S. Papin, J. Tschopp, and M. G. Grutter. 2006. Structure of the PRYSPRY-domain: implications for autoinflammatory diseases. FEBS Lett. 580:99-106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

