The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots
- PMID: 16700911
- PMCID: PMC1481516
- DOI: 10.1186/1476-4598-5-17
The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots
Abstract
Background: The genetic and molecular mechanisms responsible for and associated specifically with the development and progression of malignant prostate cells are largely unidentified. In addition, despite its implication in virtually all malignant cells, the role of altered cellular metabolism as an essential factor in prostate malignancy has been largely ignored. Moreover, the intermediary metabolism of normal prostate as well as malignant prostate cells is among the least studied and most poorly understood of all mammalian cells. Some important factors, especially the role of zinc, have been identified and implicated in the development and progression of prostate malignancy. In this review, we provide a current and updated integrated assessment of the relationships of intermediary metabolism in normal prostate and in prostate cancer. The experimental and clinical evidence that leads to the formulation of concepts of normal and malignant prostate metabolism is presented. The evidence for a concept of zinc as a tumor suppressor agent and Zip1 zinc transporter as a tumor-suppressor gene is described.
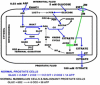
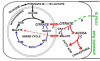
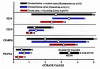
Results: The specialized function of the normal prostate glandular epithelium to produce and secrete enormously high levels of citrate involves and requires unique intermediary metabolism activities that are not generally associated with other normal mammalian cells. The accumulation of zinc by these cells is an essential factor in this unique metabolic relationship. In malignancy, the normal zinc-accumulating citrate-producing epithelial cells are metabolically transformed to citrate-oxidizing cells that lose the ability to accumulate zinc. A genetic alteration in the expression of ZIP1 zinc transporter is associated with this metabolic transformation. These genetic/metabolic relationships have important consequences on citrate-related metabolism, bioenergetics, cell proliferation and invasive capabilities of the malignant cells, which result in tumor-suppression characteristics.
Conclusion: The genetic/metabolic relationships in normal prostate glandular epithelium are driven by the unique function to accumulate and secrete citrate. The genetic/metabolic transformation of the prostate malignant cells is driven by the metabolic/bioenergetic, growth/proliferative, and invasive/migration requirements of the malignant process. Zinc is critical to these relationships. An understanding of these genetic/metabolic relationships provides new directions and opportunities for development of regimens for the prevention and treatment of prostate cancer. Important insight into the genetic/metabolic requirements of the prostate malignant process is now evolving. Most importantly at this time, an appreciation and recognition of the genetic/metabolic significance and implications in the development of prostate malignancy is imperative; and much needed research in this area is essential. Hopefully, this review will help to achieve these goals.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

