M line-deficient titin causes cardiac lethality through impaired maturation of the sarcomere
- PMID: 16702235
- PMCID: PMC2063865
- DOI: 10.1083/jcb.200601014
M line-deficient titin causes cardiac lethality through impaired maturation of the sarcomere
Abstract
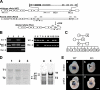
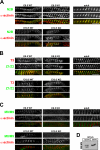
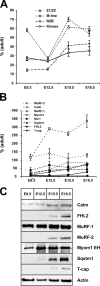
Titin, the largest protein known to date, has been linked to sarcomere assembly and function through its elastic adaptor and signaling domains. Titin's M-line region contains a unique kinase domain that has been proposed to regulate sarcomere assembly via its substrate titin cap (T-cap). In this study, we use a titin M line-deficient mouse to show that the initial assembly of the sarcomere does not depend on titin's M-line region or the phosphorylation of T-cap by the titin kinase. Rather, titin's M-line region is required to form a continuous titin filament and to provide mechanical stability of the embryonic sarcomere. Even without titin integrating into the M band, sarcomeres show proper spacing and alignment of Z discs and M bands but fail to grow laterally and ultimately disassemble. The comparison of disassembly in the developing and mature knockout sarcomere suggests diverse functions for titin's M line in embryonic development and the adult heart that not only involve the differential expression of titin isoforms but also of titin-binding proteins.
Figures




References
-
- Agarkova, I., and J.C. Perriard. 2005. The M-band: an elastic web that crosslinks thick filaments in the center of the sarcomere. Trends Cell Biol. 15:477–485. - PubMed
-
- Agarkova, I., E. Ehler, S. Lange, R. Schoenauer, and J.C. Perriard. 2003. M-band: a safeguard for sarcomere stability? J. Muscle Res. Cell Motil. 24:191–203. - PubMed
-
- Ahuja, P., E. Perriard, J.C. Perriard, and E. Ehler. 2004. Sequential myofibrillar breakdown accompanies mitotic division of mammalian cardiomyocytes. J. Cell Sci. 117:3295–3306. - PubMed
-
- Auerbach, D., B. Rothen-Ruthishauser, S. Bantle, M. Leu, E. Ehler, D. Helfman, and J.C. Perriard. 1997. Molecular mechanisms of myofibril assembly in heart. Cell Struct. Funct. 22:139–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

