Keratin 17 modulates hair follicle cycling in a TNFalpha-dependent fashion
- PMID: 16702408
- PMCID: PMC1472909
- DOI: 10.1101/gad.1387406
Keratin 17 modulates hair follicle cycling in a TNFalpha-dependent fashion
Abstract
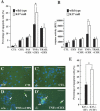
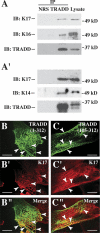
Mammalian hair follicles cycle between stages of rapid growth (anagen) and metabolic quiescence (telogen) throughout life. Transition from anagen to telogen involves an intermediate stage, catagen, consisting of a swift, apoptosis-driven involution of the lower half of the follicle. How catagen is coordinated, and spares the progenitor cells needed for anagen re-entry, is poorly understood. Keratin 17 (K17)-null mice develop alopecia in the first week post-birth, correlating with hair shaft fragility and untimely apoptosis in the hair bulb. Here we show that this abnormal apoptosis reflects premature entry into catagen. Of the proapoptotic challenges tested, K17-null skin keratinocytes in primary culture are selectively more sensitive to TNFalpha. K17 interacts with TNF receptor 1 (TNFR1)-associated death domain protein (TRADD), a death adaptor essential for TNFR1-dependent signal relay, suggesting a functional link between this keratin and TNFalpha signaling. The activity of NF-kappaB, a downstream target of TNFalpha, is increased in K17-null skin. We also find that TNFalpha is required for a timely anagen-catagen transition in mouse pelage follicles, and that its ablation partially rescues the hair cycling defect of K17-null mice. These findings identify K17 and TNFalpha as two novel and interdependent regulators of hair cycling.
Figures




References
-
- Basile J.R., Eichten A., Zacny V., Munger K. NF-κB-mediated induction of p21(Cip1/Waf1) by tumor necrosis factor α induces growth arrest and cytoprotection in normal human keratinocytes. Mol. Cancer Res. 2003;1:262–270. - PubMed
-
- Bender L.M., Morgan M.J., Thomas L.R., Liu Z.G., Thorburn A. The adaptor protein TRADD activates distinct mechanisms of apoptosis from the nucleus and the cytoplasm. Cell Death Differ. 2005;12:473–481. - PubMed
-
- Bernot K., McGowan K., Coulombe P.A. Keratin 16 expression defines a subset of epithelial cells during skin morphogenesis and the hair cycle. J. Invest. Dermatol. 2002;119:1137–1149. - PubMed
-
- Bernot K.M., Coulombe P.A., Wong P. Skin: An ideal model system to study keratin genes and proteins. Methods Cell Biol. 2004;78:453–487. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
