Developmental modulation of nonhomologous end joining in Caenorhabditis elegans
- PMID: 16702421
- PMCID: PMC1526663
- DOI: 10.1534/genetics.106.058628
Developmental modulation of nonhomologous end joining in Caenorhabditis elegans
Abstract
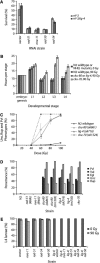
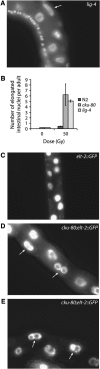
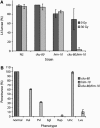
Homologous recombination and nonhomologous end joining (NHEJ) are important DNA double-strand break repair pathways in many organisms. C. elegans strains harboring mutations in the cku-70, cku-80, or lig-4 NHEJ genes displayed multiple developmental abnormalities in response to radiation-induced DNA damage in noncycling somatic cells. These phenotypes did not result from S-phase, DNA damage, or mitotic checkpoints, apoptosis, or stress response pathways that regulate dauer formation. However, an additional defect in him-10, a kinetochore component, synergized with NHEJ mutations for the radiation-induced developmental phenotypes, suggesting that they may be triggered by mis-segregation of chromosome fragments. Although NHEJ was an important DNA repair pathway for noncycling somatic cells in C. elegans, homologous recombination was used to repair radiation-induced DNA damage in cycling somatic cells and in germ cells at all times. Noncycling germ cells that depended on homologous recombination underwent cell cycle arrest in G2, whereas noncycling somatic cells that depended on NHEJ arrested in G1, suggesting that cell cycle phase may modulate DNA repair during development. We conclude that error-prone NHEJ plays little or no role in DNA repair in C. elegans germ cells, possibly ensuring homology-based double-strand break repair and transmission of a stable genome from one generation to the next.
Figures





References
-
- Ahmed, S., and J. Hodgkin, 2000. MRT-2 checkpoint protein is required for germline immortality and telomere replication in C. elegans. Nature 403: 159–164. - PubMed
-
- Ahmed, S., A. Alpi, M. O. Hengartner and A. Gartner, 2001. C. elegans RAD-5/CLK-2 defines a new DNA damage checkpoint protein. Curr. Biol. 11: 1934–1944. - PubMed
-
- Albertson, D. G., and J. N. Thomson, 1982. The kinetochores of Caenorhabditis elegans. Chromosoma 86: 409–428. - PubMed
-
- Allen, C., J. Halbrook and J. A. Nickoloff, 2003. Interactive competition between homologous recombination and non-homologous end joining. Mol. Cancer Res. 1: 913–920. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

