Vascular endothelial growth factor is an important determinant of sepsis morbidity and mortality
- PMID: 16702604
- PMCID: PMC2118329
- DOI: 10.1084/jem.20060375
Vascular endothelial growth factor is an important determinant of sepsis morbidity and mortality
Abstract
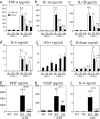
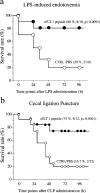
Sepsis, the systemic inflammatory response to infection, is a leading cause of morbidity and mortality. The mechanisms of sepsis pathophysiology remain obscure but are likely to involve a complex interplay between mediators of the inflammatory and coagulation pathways. An improved understanding of these mechanisms should provide an important foundation for developing novel therapies. In this study, we show that sepsis is associated with a time-dependent increase in circulating levels of vascular endothelial growth factor (VEGF) and placental growth factor (PlGF) in animal and human models of sepsis. Adenovirus-mediated overexpression of soluble Flt-1 (sFlt-1) in a mouse model of endotoxemia attenuated the rise in VEGF and PlGF levels and blocked the effect of endotoxemia on cardiac function, vascular permeability, and mortality. Similarly, in a cecal ligation puncture (CLP) model, adenovirus-sFlt-1 protected against cardiac dysfunction and mortality. When administered in a therapeutic regimen beginning 1 h after the onset of endotoxemia or CLP, sFlt peptide resulted in marked improvement in cardiac physiology and survival. Systemic administration of antibodies against the transmembrane receptor Flk-1 but not Flt-1 protected against sepsis mortality. Adenovirus-mediated overexpression of VEGF but not PlGF exacerbated the lipopolysaccharide-mediated toxic effects. Together, these data support a pathophysiological role for VEGF in mediating the sepsis phenotype.
Figures








References
-
- Aird, W.C. 2003. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 101:3765–3777. - PubMed
-
- Angus, D.C., W.T. Linde-Zwirble, J. Lidicker, G. Clermont, J. Carcillo, and M.R. Pinsky. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29:1303–1310. - PubMed
-
- Panacek, E.A., J.C. Marshall, T.E. Albertson, D.H. Johnson, S. Johnson, R.D. MacArthur, M. Miller, W.T. Barchuk, S. Fischkoff, M. Kaul, et al. 2004. Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F(ab')2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit. Care Med. 32:2173–2182. - PubMed
-
- Bernard, G.R., J.L. Vincent, P.F. Laterre, S.P. LaRosa, J.F. Dhainaut, A. Lopez-Rodriguez, J.S. Steingrub, G.E. Garber, J.D. Helterbrand, E.W. Ely, and C.J. Fisher Jr. 2001. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 344:699–709. - PubMed
-
- Senger, D.R., S.J. Galli, A.M. Dvorak, C.A. Perruzzi, V.S. Harvey, and H.F. Dvorak. 1983. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 219:983–985. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

