Mechanism of action of novel NO-releasing furoxan derivatives of aspirin in human platelets
- PMID: 16702997
- PMCID: PMC1751793
- DOI: 10.1038/sj.bjp.0706743
Mechanism of action of novel NO-releasing furoxan derivatives of aspirin in human platelets
Abstract
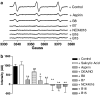
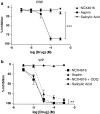
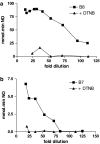
Incorporation of a nitric oxide (NO)-releasing moiety in aspirin can overcome its gastric side effects. We investigated the NO-release patterns and antiplatelet effects of novel furoxan derivatives of aspirin (B8 and B7) in comparison to existing antiplatelet agents. Cyclooxygenase (COX) activity was investigated in purified enzyme using an electron paramagnetic resonance-based technique. Concentration-response curves for antiplatelet agents +/- the soluble guanylate cyclase inhibitor, ODQ (50 microM) were generated in platelet-rich plasma (PRP) and washed platelets (WP) activated with collagen using turbidometric aggregometry. NO was detected using an isolated NO electrode. The furoxan derivatives of aspirin (B8, B7) and their NO-free furazan equivalents (B16, B15; all 100 microM) significantly inhibited COX activity (P < 0.01; n = 6) in vitro and caused aspirin-independent, cGMP-dependent inhibition of collagen-induced platelet aggregation in WP. B8 was more potent than B7 (PRP IC(50) = 0.62 +/- 0.1 microM for B8; 400 +/- 89 microM for B7; P < 0.0001. WP IC(50)s = 0.6 +/- 0.1 and 62 +/- 10 microM, respectively). The NO-free furazan counterparts were less potent antiplatelet agents (WP IC(50)s = 54 +/- 3 microM and 62 +/- 10 microM, respectively; P < 0.0001, B8 vs B16). Of the hybrids investigated, only B8 retained antiplatelet activity in PRP.NO release from furoxan-aspirin hybrids was undetectable in buffer alone, but was accelerated in the presence of either plasma or plasma components, albumin (4%), glutathione (GSH; 3 microM) and ascorbate (50 microM), the effects of which were additive for B7 but not B8. NO generation from furoxans was greatly enhanced by platelet extract, an effect that could largely be explained by the synergistic effect of intracellular concentrations of GSH (3 mM) and ascorbate (1 mM). We conclude that the decomposition of furoxan-aspirin hybrids to generate biologically active NO is catalysed by endogenous agents which may instil a potential for primarily intracellular delivery of NO. The blunting of the aspirin effects of furoxan hybrids is likely to be due to loss of the acetyl moiety in plasma; the observed antiplatelet effects are thereby primarily mediated via NO release. Compounds of this class might represent a novel means of inhibiting platelet aggregation by a combination of NO generation and COX inhibition.
Figures


References
-
- ANDERSON T.J., GERHARD M.D., MEREDITH I.T., CHARBONNEAU F., DELAGRANGE D., CREAGER M.A., SELWYN A.P., GANZ P. Systemic nature of endothelial dysfunction in atherosclerosis. Am. J. Cardiol. 1995;75:71B–74B. - PubMed
-
- BROWN J.F., KEATES A.C., HANSON P.J., WHITTLE B.J. Nitric oxide generators and cGMP stimulate mucus secretion by rat gastric mucosal cells. Am. J. Physiol. 1993;265:G418–G422. - PubMed
-
- CAMERON A.J. Aspirin and gastric ulcer. Mayo Clin. Proc. 1975;50:565–570. - PubMed
-
- CENA C., LOLLI M.L., LAZZARATO L., GUAITA E., MORINI G., CORUZZI G., MCELROY S.P., MEGSON I.L., FRUTTERO R., GASCO A. Antiinflammatory, gastrosparing, and antiplatelet properties of new NO-donor esters of aspirin. J. Med. Chem. 2003;46:747–754. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

