The boundary vector cell model of place cell firing and spatial memory
- PMID: 16703944
- PMCID: PMC2677716
- DOI: 10.1515/revneuro.2006.17.1-2.71
The boundary vector cell model of place cell firing and spatial memory
Abstract
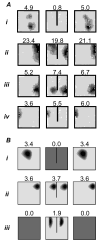
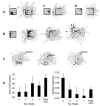
We review evidence for the boundary vector cell model of the environmental determinants of the firing of hippocampal place cells. Preliminary experimental results are presented concerning the effects of addition or removal of environmental boundaries on place cell firing and evidence that boundary vector cells may exist in the subiculum. We review and update computational simulations predicting the location of human search within a virtual environment of variable geometry, assuming that boundary vector cells provide one of the input representations of location used in mammalian spatial memory. Finally, we extend the model to include experience-dependent modification of connection strengths through a BCM-like learning rule - the size and sign of strength change is influenced by historic activity of the postsynaptic cell. Simulations are compared to experimental data on the firing of place cells under geometrical manipulations to their environment. The relationship between neurophysiological results in rats and spatial behaviour in humans is discussed.
Figures








References
-
- O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. - PubMed
-
- O'Keefe J. Place units in the hippocampus of the freely moving rat. Exp Neurol. 1976;51:78–109. - PubMed
-
- Burwell RD, Witter MP, Amaral DG. Perirhinal and postrhinal cortices of the rat: a review of the neuroanatomical literature and comparison with findings from the monkey brain. Hippocampus. 1995;6:390–408. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
