Artemether-lumefantrine versus artesunate plus amodiaquine for treating uncomplicated childhood malaria in Nigeria: randomized controlled trial
- PMID: 16704735
- PMCID: PMC1475595
- DOI: 10.1186/1475-2875-5-43
Artemether-lumefantrine versus artesunate plus amodiaquine for treating uncomplicated childhood malaria in Nigeria: randomized controlled trial
Abstract
Background: The therapeutic efficacy of artesunate plus amodiaquine and artemether/lumefantrine were assessed in an area of Nigeria with high levels of Plasmodium falciparum resistance to chloroquine and sulphadoxine-pyrimethamine.
Participants: Children aged 6 to 59 months with uncomplicated P. falciparum infection and parasite density 1,000 to 200,000 parasites/microL enrolled following informed consent by parents.
Methods: Eligible children were randomly assigned to receive either a 3-day course of artesunate (4 mg/kg) plus amodiaquine (10 mg/kg) or 6-dose course of artemether/lumefantrine (20/120 mg tablets) over three days. Patients were followed up with clinical and laboratory assessments until day 14 using standard WHO in-vivo antimalarial drug test protocol.
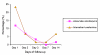
Results: A total 119 eligible children were enrolled but 111 completed the study. Adequate clinical and parasitological response (ACPR) was 47 (87.0%) and 47 (82.5%) for artemether-lumefantrine (AL) and artesunate+amodiaquine (AAMQ) respectively (OR 0.7, 95% confidence interval 0.22 to 2.22). Early treatment failure (ETF) occurred in one participant (1.8%) treated with AAQ but in none of those with AL. Two (3.7%) patients in the AL group and none in the AAQ group had late clinical failure. Late parasitological failure was observed in 9 (15.8) and 5 (9.3%) of patients treated with AAQ and AL respectively. None of participants had a serious adverse event.
Conclusion: Artemether-lumenfantrine and artesunate plus amodiaquine have high and comparable cure rates and tolerability among under-five children in Calabar, Nigeria.
Figures
References
-
- WHO . A global strategy for malaria control. Geneva: World Health Organization; 1993.
-
- Ezedinachi ENU, Alaribe AA, Meremikwu M, Ejezie GC. New trends in chloroquine efficacy in the treatment of malaria. Central Afr J Med. 1992;38:303–307. - PubMed
-
- Ekanem OJ, Ezedinachi ENU, Molta NB, Watila IM, Chukwuani CM, Meremikwu MM, Akpede G, Ojar EA. Treatment of malaria in North-Eastern and South-Eastern Nigeria: a population study of mefloquine, sulphadoxine, pyrimethamine combination (MSP) vs chloroquine. West Afr J Med. 2000;19:293–297. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources