Protein phosphatase 2A stabilizes human securin, whose phosphorylated forms are degraded via the SCF ubiquitin ligase
- PMID: 16705156
- PMCID: PMC1489102
- DOI: 10.1128/MCB.01904-05
Protein phosphatase 2A stabilizes human securin, whose phosphorylated forms are degraded via the SCF ubiquitin ligase
Abstract
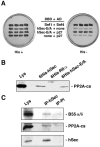
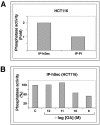
Sister chromatid segregation is triggered at the metaphase-to-anaphase transition by the activation of the protease separase. For most of the cell cycle, separase activity is kept in check by its association with the inhibitory chaperone securin. Activation of separase occurs at anaphase onset, when securin is targeted for destruction by the anaphase-promoting complex or cyclosome E3 ubiquitin protein ligase. This results in the release of the cohesins from chromosomes, which in turn allows the segregation of sister chromatids to opposite spindle poles. Here we show that human securin (hSecurin) forms a complex with enzymatically active protein phosphatase 2A (PP2A) and that it is a substrate of the phosphatase, both in vitro and in vivo. Treatment of cells with okadaic acid, a potent inhibitor of PP2A, results in various hyperphosphorylated forms of hSecurin which are extremely unstable, due to the action of the Skp1/Cul1/F-box protein complex ubiquitin ligase. We propose that PP2A regulates hSecurin levels by counteracting its phosphorylation, which promotes its degradation. Misregulation of this process may lead to the formation of tumors, in which overproduction of hSecurin is often observed.
Figures




References
-
- Bernal, J. A., R. Luna, A. Espina, I. Lazaro, F. Ramos-Morales, F. Romero, C. Arias, A. Silva, M. Tortolero, and J. A. Pintor-Toro. 2002. Human securin interacts with p53 and modulates p53-mediated transcriptional activity and apoptosis. Nat. Genet. 32:306-311. - PubMed
-
- Bhatia, K., K. Huppi, G. Spangler, D. Siwarski, R. Iyer, and I. Magrath. 1993. Point mutations in the c-Myc transactivation domain are common in Burkitt's lymphoma and mouse plasmacytomas. Nat. Genet. 5:56-61. - PubMed
-
- Breeden, L., and K. Nasmyth. 1985. Regulation of the yeast HO gene. Quant. Biol. 50:643-650. - PubMed
-
- Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550-553. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
