T-cell protein tyrosine phosphatase (Tcptp) is a negative regulator of colony-stimulating factor 1 signaling and macrophage differentiation
- PMID: 16705167
- PMCID: PMC1489091
- DOI: 10.1128/MCB.01932-05
T-cell protein tyrosine phosphatase (Tcptp) is a negative regulator of colony-stimulating factor 1 signaling and macrophage differentiation
Abstract
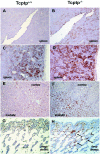
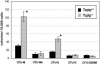
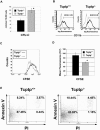
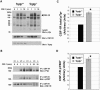
Mice null for the T-cell protein tyrosine phosphatase (Tcptp-/-) die shortly after birth due to complications arising from the development of a systemic inflammatory disease. It was originally reported that Tcptp-/- mice have increased numbers of macrophages in the spleen; however, the mechanism underlying the aberrant growth and differentiation of macrophages in Tcptp-/- mice is not known. We have identified Tcptp as an important regulator of colony-stimulating factor 1 (CSF-1) signaling and mononuclear phagocyte development. The number of CSF-1-dependent CFU is increased in Tcptp-/- bone marrow. Tcptp-/- mice also have increased numbers of granulocyte-macrophage precursors (GMP), and these Tcptp-/- GMP yield more macrophage colonies in response to CSF-1 relative to wild-type cells. Furthermore, we have identified the CSF-1 receptor (CSF-1R) as a physiological target of Tcptp through substrate-trapping experiments and its hyperphosphorylation in Tcptp-/- macrophages. Tcptp-/- macrophages also have increased tyrosine phosphorylation and recruitment of a Grb2/Gab2/Shp2 complex to the CSF-1R and enhanced activation of Erk after CSF-1 stimulation, which are important molecular events in CSF-1-induced differentiation. These data implicate Tcptp as a critical regulator of CSF-1 signaling and mononuclear phagocyte development in hematopoiesis.
Figures



References
-
- Alberola-Ila, J., and G. Hernandez-Hoyos. 2003. The Ras/MAPK cascade and the control of positive selection. Immunol. Rev. 191:79-96. - PubMed
-
- Alessi, D. R., S. R. James, C. P. Downes, A. B. Holmes, P. R. Gaffney, C. B. Reese, and P. Cohen. 1997. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr. Biol. 7:261-269. - PubMed
-
- Austyn, J. M., and S. Gordon. 1981. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur. J. Immunol. 11:805-815. - PubMed
-
- Bartelmez, S. H., T. R. Bradley, I. Bertoncello, D. Y. Mochizuki, R. J. Tushinski, E. R. Stanley, A. J. Hapel, I. G. Young, A. B. Kriegler, and G. S. Hodgson. 1989. Interleukin 1 plus interleukin 3 plus colony-stimulating factor 1 are essential for clonal proliferation of primitive myeloid bone marrow cells. Exp. Hematol. 17:240-245. - PubMed
-
- Berg, K. L., K. A. Siminovitch, and E. R. Stanley. 1999. SHP-1 regulation of p62(DOK) tyrosine phosphorylation in macrophages. J. Biol. Chem. 274:35855-35865. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
