N-cadherin and keratinocyte growth factor receptor mediate the functional interplay between Ki-RASG12V and p53V143A in promoting pancreatic cell migration, invasion, and tissue architecture disruption
- PMID: 16705170
- PMCID: PMC1489079
- DOI: 10.1128/MCB.01055-05
N-cadherin and keratinocyte growth factor receptor mediate the functional interplay between Ki-RASG12V and p53V143A in promoting pancreatic cell migration, invasion, and tissue architecture disruption
Abstract
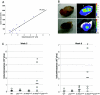
The genetic basis of pancreatic ductal adenocarcinoma, which constitutes the most common type of pancreatic malignancy, involves the sequential activation of oncogenes and inactivation of tumor suppressor genes. Among the pivotal genetic alterations are Ki-RAS oncogene activation and p53 tumor suppressor gene inactivation. We explain that the combination of these genetic events facilitates pancreatic carcinogenesis as revealed in novel three-dimensional cell (spheroid cyst) culture and in vivo subcutaneous and orthotopic xenotransplantation models. N-cadherin, a member of the classic cadherins important in the regulation of cell-cell adhesion, is induced in the presence of Ki-RAS mutation but subsequently downregulated with the acquisition of p53 mutation as revealed by gene microarrays and corroborated by reverse transcription-PCR and Western blotting. N-cadherin modulates the capacity of pancreatic ductal cells to migrate and invade, in part via complex formation with keratinocyte growth factor receptor and neural cell adhesion molecule and in part via interaction with p120-catenin. However, modulation of these complexes by Ki-RAS and p53 leads to enhanced cell migration and invasion. This preferentially induces the downstream effector AKT over mitogen-activated protein kinase to execute changes in cellular behavior. Thus, we are able to define molecules that in part are directly affected by Ki-RAS and p53 during pancreatic ductal carcinogenesis, and this provides a platform for potential new molecularly based therapeutic interventions.
Figures
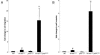







References
-
- Agbunag, C., and D. Bar-Sagi. 2004. Oncogenic K-ras drives cell cycle progression and phenotypic conversion of primary pancreatic duct epithelial cells. Cancer Res. 64:5659-5663. - PubMed
-
- Aguirre, A. J., C. Brennan, G. Bailey, R. Sinha, B. Feng, C. Leo, Y. Zhang, J. Zhang, J. D. Gans, N. Bardeesy, C. Cauwels, C. Cordon-Cardo, M. S. Redston, R. A. DePinho, and L. Chin. 2004. High-resolution characterization of the pancreatic adenocarcinoma genome. Proc. Natl. Acad. Sci. USA 101:9067-9072. - PMC - PubMed
-
- Alencar, H., R. King, M. Funovics, C. Stout, R. Weissleder, and U. Mahmood. 2005. A novel mouse model for segmental orthotopic colon cancer. Int. J. Cancer 117:335-339. - PubMed
-
- Almoguera, C., D. Shibata, K. Forrester, J. Martin, N. Arnheim, and M. Perucho. 1988. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell 53:549-554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
