E Proteins and Id2 converge on p57Kip2 to regulate cell cycle in neural cells
- PMID: 16705184
- PMCID: PMC1489106
- DOI: 10.1128/MCB.01743-05
E Proteins and Id2 converge on p57Kip2 to regulate cell cycle in neural cells
Abstract
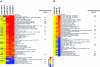
A precise balance between proliferation and differentiation must be maintained during neural development to obtain the correct proportion of differentiated cell types in the adult nervous system. The basic helix-loop-helix (bHLH) transcription factors known as E proteins and their natural inhibitors, the Id proteins, control the timing of differentiation and terminal exit from the cell cycle. Here we show that progression into S phase of human neuroblastoma cells is prevented by E proteins and promoted by Id2. Cyclin-dependent kinase inhibitors (CKI) have been identified as key effectors of cell cycle arrest in differentiating cells. However, p57Kip2 is the only CKI that is absolutely required for normal development. Through the use of global gene expression analysis in neuroblastoma cells engineered to acutely express the E protein E47 and Id2, we find that p57Kip2 is a target of E47. Consistent with the role of Id proteins, Id2 prevents activation of p57Kip2 expression, and the retinoblastoma tumor suppressor protein, a known Id2 inhibitor, counters this activity. The strong E47-mediated inhibition of entry into S phase is entirely reversed in cells in which expression of p57Kip2 is silenced by RNA interference. During brain development, expression of p57Kip2 is opposite that of Id2. Our findings identify p57Kip2 as a functionally relevant target recruited by bHLH transcription factors to induce cell cycle arrest in developing neuroblasts and suggest that deregulated expression of Id proteins may be an epigenetic mechanism to silence expression of this CKI in neural tumors.
Figures
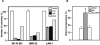







Similar articles
-
FHL2 interacts with and acts as a functional repressor of Id2 in human neuroblastoma cells.Nucleic Acids Res. 2009 Jul;37(12):3996-4009. doi: 10.1093/nar/gkp332. Epub 2009 May 5. Nucleic Acids Res. 2009. PMID: 19417068 Free PMC article.
-
The protein ENH is a cytoplasmic sequestration factor for Id2 in normal and tumor cells from the nervous system.Proc Natl Acad Sci U S A. 2006 Mar 28;103(13):4976-81. doi: 10.1073/pnas.0600168103. Epub 2006 Mar 20. Proc Natl Acad Sci U S A. 2006. PMID: 16549780 Free PMC article.
-
p57Kip2 is a repressor of Mash1 activity and neuronal differentiation in neural stem cells.Cell Death Differ. 2009 Sep;16(9):1256-65. doi: 10.1038/cdd.2009.72. Epub 2009 Jul 10. Cell Death Differ. 2009. PMID: 19590511
-
Stem cells, differentiation and cell cycle control in pituitary.Front Horm Res. 2010;38:15-24. doi: 10.1159/000318490. Epub 2010 Jul 5. Front Horm Res. 2010. PMID: 20616491 Review.
-
Inhibitors of DNA binding in neural cell proliferation and differentiation.Neurochem Res. 2003 Jan;28(1):45-52. doi: 10.1023/a:1021691911011. Neurochem Res. 2003. PMID: 12587662 Review.
Cited by
-
MyoD regulates p57kip2 expression by interacting with a distant cis-element and modifying a higher order chromatin structure.Nucleic Acids Res. 2012 Sep 1;40(17):8266-75. doi: 10.1093/nar/gks619. Epub 2012 Jun 26. Nucleic Acids Res. 2012. PMID: 22740650 Free PMC article.
-
The Roles of Antisense Long Noncoding RNAs in Tumorigenesis and Development through Cis-Regulation of Neighbouring Genes.Biomolecules. 2023 Apr 18;13(4):684. doi: 10.3390/biom13040684. Biomolecules. 2023. PMID: 37189431 Free PMC article. Review.
-
Distinct effects of Hedgehog signaling on neuronal fate specification and cell cycle progression in the embryonic mouse retina.J Neurosci. 2009 May 27;29(21):6932-44. doi: 10.1523/JNEUROSCI.0289-09.2009. J Neurosci. 2009. PMID: 19474320 Free PMC article.
-
N-(4-Hydroxyphenyl) retinamide potentiated paclitaxel for cell cycle arrest and apoptosis in glioblastoma C6 and RG2 cells.Brain Res. 2009 May 1;1268:142-153. doi: 10.1016/j.brainres.2009.02.064. Epub 2009 Mar 10. Brain Res. 2009. PMID: 19285047 Free PMC article.
-
Inhibitor of DNA binding proteins revealed as orchestrators of steady state, stress and malignant hematopoiesis.Front Immunol. 2022 Aug 5;13:934624. doi: 10.3389/fimmu.2022.934624. eCollection 2022. Front Immunol. 2022. PMID: 35990659 Free PMC article. Review.
References
-
- Alheim, K., J. Corness, M. K. Samuelsson, L. G. Bladh, T. Murata, T. Nilsson, and S. Okret. 2003. Identification of a functional glucocorticoid response element in the promoter of the cyclin-dependent kinase inhibitor p57Kip2. J. Mol. Endocrinol. 30:359-368. - PubMed
-
- Bain, G., and C. Murre. 1998. The role of E-proteins in B- and T-lymphocyte development. Semin. Immunol. 10:143-153. - PubMed
-
- Benezra, R., R. L. Davis, D. Lockshon, D. L. Turner, and H. Weintraub. 1990. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell 61:49-59. - PubMed
-
- Chung, W. Y., L. Yuan, L. Feng, T. Hensle, and B. Tycko. 1996. Chromosome 11p15.5 regional imprinting: comparative analysis of KIP2 and H19 in human tissues and Wilms' tumors. Hum. Mol. Genet. 5:1101-1108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
