Alterations in expression and chromatin configuration of the alpha hemoglobin-stabilizing protein gene in erythroid Kruppel-like factor-deficient mice
- PMID: 16705186
- PMCID: PMC1489081
- DOI: 10.1128/MCB.02216-05
Alterations in expression and chromatin configuration of the alpha hemoglobin-stabilizing protein gene in erythroid Kruppel-like factor-deficient mice
Abstract
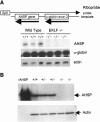
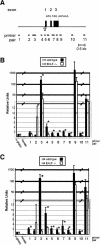
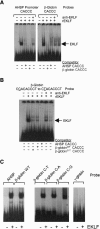
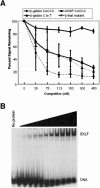
Erythroid Krüppel-like factor (EKLF) is an erythroid zinc finger protein identified by its interaction with a CACCC sequence in the beta-globin promoter, where it establishes local chromatin structure permitting beta-globin gene transcription. We sought to identify other EKLF target genes and determine the chromatin status of these genes in the presence and absence of EKLF. We identified alpha hemoglobin-stabilizing protein (AHSP) by subtractive hybridization and demonstrated a 95 to 99.9% reduction in AHSP mRNA and the absence of AHSP in EKLF-deficient cells. Chromatin at the AHSP promoter from EKLF-deficient cells lacked a DNase I hypersensitive site and exhibited histone hypoacetylation across the locus compared to hyperacetylation of wild-type chromatin. Wild-type chromatin demonstrated a peak of EKLF binding over a promoter region CACCC box that differs from the EKLF consensus by a nucleotide. In mobility shift assays, the AHSP promoter CACCC site bound EKLF in a manner comparable to the beta-globin promoter CACCC site, indicating a broader recognition sequence for the EKLF consensus binding site. The AHSP promoter was transactivated by EKLF in K562 cells, which lack EKLF. These results support the hypothesis that EKLF acts as a transcription factor and a chromatin modulator for the AHSP and beta-globin genes and indicate that EKLF may play similar roles for other erythroid genes.
Figures




References
-
- Armstrong, J. A., J. J. Bieker, and B. M. Emerson. 1998. A SWI/SNF-related chromatin remodeling complex, E-RC1, is required for tissue-specific transcriptional regulation by EKLF in vitro. Cell 95:93-104. - PubMed
-
- Asano, H., X. S. Li, and G. Stamatoyannopoulos. 2000. FKLF-2: a novel Kruppel-like transcriptional factor that activates globin and other erythroid lineage genes. Blood 95:3578-3584. - PubMed
-
- Basu, P., T. G. Sargent, L. C. Redmond, J. C. Aisenberg, E. P. Kransdorf, S. Z. Wang, G. D. Ginder, and J. A. Lloyd. 2004. Evolutionary conservation of KLF transcription factors and functional conservation of human gamma-globin gene regulation in chicken. Genomics 84:311-319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
