Controlled clinical trials in cancer pain. How controlled should they be? A qualitative systematic review
- PMID: 16705312
- PMCID: PMC2361312
- DOI: 10.1038/sj.bjc.6603162
Controlled clinical trials in cancer pain. How controlled should they be? A qualitative systematic review
Abstract
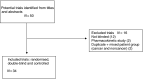
This qualitative systematic review of the clinical methodology used in randomised, controlled trials of oral opioids (morphine, hydromorphone, oxycodone) for cancer pain underlines the difficulties of good pain research in palliative care. The current literature lacks placebo-controlled superiority trials. Recommendations for future research are discussed.
References
-
- Antczak AA, Tang J, Chalmers DC (1986a) Quality assessment of randomized control trials in dental research. I. Methods. J Periodontal Res 21: 305–314 - PubMed
-
- Antczak AA, Tang J, Chalmers DC (1986b) Quality assessment of randomized control trials in dental research. II. Results: periodontal research. J Periodontal Res 21: 315–321 - PubMed
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17: 1–12 - PubMed
-
- Keefe FJ, Abernethy AP, Campbell LC (2005) Psychological approaches to understanding and treating disease-related pain. Annu Rev Psychol 56: 601–630 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical