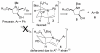A concise stereoselective synthesis of Preussin, 3-epi-Preussin, and analogues
- PMID: 16706524
- PMCID: PMC2613785
- DOI: 10.1021/ol0606435
A concise stereoselective synthesis of Preussin, 3-epi-Preussin, and analogues
Abstract
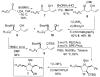
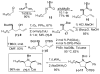
[reaction: see text] A new stereoselective synthesis of the antifungal and antitumor agents Preussin and 3-epi-Preussin via a Pd-catalyzed carboamination of a protected amino alcohol is described. The key transformation leads to simultaneous formation of the N-C2 bond and the C1'-aryl bond, and allows installation of the aryl group one step from the end of the sequence. This strategy permits the facile construction of a variety of preussin analogues bearing different aromatic groups.
Figures
References
-
- Schwartz RE, Liesch J, Hensens O, Zitano L, Honeycutt S, Garrity G, Fromtling RA, Onishi J, Monaghan R. J. Antibiot. 1988;41:1774. - PubMed
-
- Kinzy TG, Harger JW, Carr-Schmid A, Kwon J, Shastry M, Justice M, Dinman JD. Virology. 2002;300:60. - PubMed
-
- Okue M, Watanabe H, Kasahara K, Yoshida M, Horinouchi S, Kitahara T. Biosci. Biotechnol. Biochem. 2002;66:1093. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous