Inhibition of dsRNA-induced signaling in hepatitis C virus-infected cells by NS3 protease-dependent and -independent mechanisms
- PMID: 16707574
- PMCID: PMC1482521
- DOI: 10.1073/pnas.0602957103
Inhibition of dsRNA-induced signaling in hepatitis C virus-infected cells by NS3 protease-dependent and -independent mechanisms
Abstract
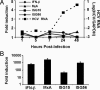
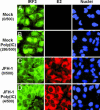
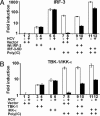
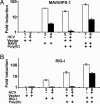
The recent establishment of a robust hepatitis C virus (HCV) cell culture system permits analysis of virus-host interactions during HCV infection. Here, we report that HCV genotype 2a (JFH-1) infection fails to induce IFN-beta or IFN-stimulated gene expression in Huh-7 cells, and that it blocks IFN-beta and IFN-stimulated gene production after transfection of synthetic dsRNA. Overexpression of individual components of the dsRNA-signaling pathway in HCV-infected and uninfected cells indicates that HCV inhibits IFN-beta promoter activity by inactivating the mitochondrial antiviral signaling protein/IFN-beta promoter stimulator 1 (MAVS/IPS-1), while leaving the IFN-induced Janus kinases-signal transducers and activators of transcription (JAK-STAT) signaling pathway intact. We also show that MAVS/IPS-1-dependent IFN-beta promoter activity in HCV-infected cells is fully restored by the nonstructural protein 3 (NS3) protease inhibitor BILN2061. In contrast, synthetic dsRNA-induced IFN-beta promoter activity is not restored by BILN2061, although it is partially restored by overexpression of RIG-I. These results support recently reported evidence that the HCV NS3 protease blunts the ability of HCV to induce IFN-beta promoter activity by proteolytically cleaving MAVS/IPS-1. The results also suggest that HCV blocks the synthetic dsRNA-induced signaling pathway at a point upstream of MAVS/IPS-1, and that it does so by an NS3-independent mechanism.
Conflict of interest statement
Conflict of interest statement: F.V.C. consults for several companies that are developing antiviral drugs and/or vaccines for HCV.
Figures
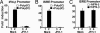
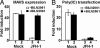
References
-
- Hoofnagle J. H. Hepatology. 2002;36:S21–S29. - PubMed
-
- Alter H. J., Seeff L. B. Semin. Liver Dis. 2000;20:17–35. - PubMed
-
- Lindenbach B. D., Evans M. J., Syder A. J., Wolk B., Tellinghuisen T. L., Liu C. C., Maruyama T., Hynes R. O., Burton D. R., McKeating J. A., Rice C. M. Science. 2005;309:623–626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

