Attachment of capsular polysaccharide to the cell wall of Streptococcus pneumoniae type 2 is required for invasive disease
- PMID: 16707578
- PMCID: PMC1482522
- DOI: 10.1073/pnas.0602148103
Attachment of capsular polysaccharide to the cell wall of Streptococcus pneumoniae type 2 is required for invasive disease
Abstract
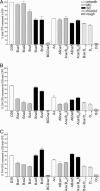
The capacity of Streptococcus pneumoniae to produce capsular polysaccharide (CPS) is essential for virulence. The CPS biosynthesis proteins CpsB, CpsC, and CpsD function to regulate CPS production via tyrosine phosphorylation of CpsD. This mechanism of regulating CPS production is important for enabling S. pneumoniae to cause invasive disease. Here, we identify mutations affecting the attachment of CPS to the cell wall. These mutations were located in cpsC, such that CpsC functioned independently from CpsD tyrosine phosphorylation. These mutants produced WT levels of CPS, but were unable to cause bacteremia in mice after intranasal challenge. This finding suggests that cell-wall attachment of CPS is essential for invasive pneumococcal disease; production of WT levels of CPS alone is not sufficient. We also show that cpsB mutants, which lack the phosphotyrosine-protein phosphatase, produced less CPS than the WT strain, but attached substantially more CPS to their cell wall. Thus, the phosphorylated form of CpsD promotes attachment of CPS to the cell wall.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

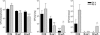

References
-
- Austrian R. Rev. Infect. Dis. 1981;3(Suppl):S1–S17. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

