Circadian rhythms in gene transcription imparted by chromosome compaction in the cyanobacterium Synechococcus elongatus
- PMID: 16707582
- PMCID: PMC1482530
- DOI: 10.1073/pnas.0508696103
Circadian rhythms in gene transcription imparted by chromosome compaction in the cyanobacterium Synechococcus elongatus
Abstract
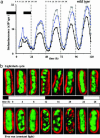
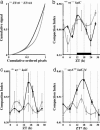
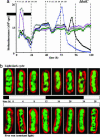
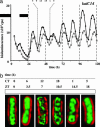
In the cyanobacterium Synechococcus elongatus (PCC 7942) the kai genes A, B, and C and the sasA gene encode the functional protein core of the timing mechanism essential for circadian clock regulation of global gene expression. The Kai proteins comprise the central timing mechanism, and the sensor kinase SasA is a primary transducer of temporal information. We demonstrate that the circadian clock also regulates a chromosome compaction rhythm. This chromosome compaction rhythm is both circadian clock-controlled and kai-dependent. Although sasA is required for global gene expression rhythmicity, it is not required for these chromosome compaction rhythms. We also demonstrate direct control by the Kai proteins on the rate at which the SasA protein autophosphorylates. Thus, to generate and maintain circadian rhythms in gene expression, the Kai proteins keep relative time, communicate temporal information to SasA, and may control access to promoter elements by imparting rhythmic chromosome compaction.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


References
-
- Ditty J. L., Williams S. B., Golden S. S. Annu. Rev. Genet. 2003;37:513–543. - PubMed
-
- Dunlap J. C., Loros J. J. J. Biol. Rhythms. 2004;19:414–424. - PubMed
-
- Young M. W., Kay S. A. Nat. Rev. Genet. 2001;2:702–715. - PubMed
-
- Nakajima M., Imai K., Ito H., Nishiwaki T., Murayama Y., Iwasaki H., Oyama T., Kondo T. Science. 2005;308:414–415. - PubMed
-
- Tomita J., Nakajima M., Kondo T., Iwasaki H. Science. 2005;307:251–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

