Multiple start codons and phosphorylation result in discrete Rad52 protein species
- PMID: 16707661
- PMCID: PMC1463902
- DOI: 10.1093/nar/gkl280
Multiple start codons and phosphorylation result in discrete Rad52 protein species
Abstract
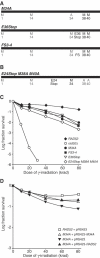
The sequence of the Saccharomyces cerevisiae RAD52 gene contains five potential translation start sites and protein-blot analysis typically detects multiple Rad52 species with different electrophoretic mobilities. Here we define the gene products encoded by RAD52. We show that the multiple Rad52 protein species are due to promiscuous choice of start codons as well as post-translational modification. Specifically, Rad52 is phosphorylated both in a cell cycle-independent and in a cell cycle-dependent manner. Furthermore, phosphorylation is dependent on the presence of the Rad52 C terminus, but not dependent on its interaction with Rad51. We also show that the Rad52 protein can be translated from the last three start sites and expression from any one of them is sufficient for spontaneous recombination and the repair of gamma-ray-induced double-strand breaks.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

