Urocortin 2-deficient mice exhibit gender-specific alterations in circadian hypothalamus-pituitary-adrenal axis and depressive-like behavior
- PMID: 16707802
- PMCID: PMC6675306
- DOI: 10.1523/JNEUROSCI.3955-05.2006
Urocortin 2-deficient mice exhibit gender-specific alterations in circadian hypothalamus-pituitary-adrenal axis and depressive-like behavior
Abstract
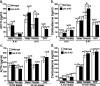
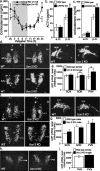
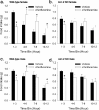
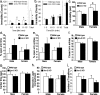
Gender differences in hypothalamus-pituitary-adrenal (HPA) axis activation and the prevalence of mood disorders are well documented. Urocortin 2, a recently identified member of the corticotropin-releasing factor family, is expressed in discrete neuroendocrine and stress-related nuclei of the rodent CNS. To determine the physiological role of urocortin 2, mice null for urocortin 2 were generated and HPA axis activity, ingestive, and stress-related behaviors and alterations in expression levels of CRF-related ligands and receptors were examined. Here we report that female, but not male, mice lacking urocortin 2 exhibit a significant increase in the basal daily rhythms of ACTH and corticosterone and a significant decrease in fluid intake and depressive-like behavior. The differential phenotype of urocortin 2 deficiency in female and male mice may imply a role for urocortin 2 in these gender differences.
Figures




References
-
- Amat J, Tamblyn JP, Paul ED, Bland ST, Amat P, Foster AC, Watkins LR, Maier SF (2004). Microinjection of urocortin 2 into the dorsal raphe nucleus activates serotonergic neurons and increases extracellular serotonin in the basolateral amygdala. Neuroscience 129:509–519. - PubMed
-
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB (1999). The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol 160:1–12. - PubMed
-
- Bale TL, Vale WW (2004). CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol 44:525–557. - PubMed
-
- Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF (2000). Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet 24:410–414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
