Early life blockade of 5-hydroxytryptamine 1A receptors normalizes sleep and depression-like behavior in adult knock-out mice lacking the serotonin transporter
- PMID: 16707806
- PMCID: PMC6675294
- DOI: 10.1523/JNEUROSCI.5156-05.2006
Early life blockade of 5-hydroxytryptamine 1A receptors normalizes sleep and depression-like behavior in adult knock-out mice lacking the serotonin transporter
Abstract
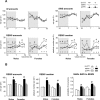
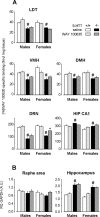
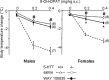
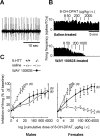
In serotonin transporter knock-out (5-HTT-/-) mice, extracellular serotonin (5-HT) levels are markedly elevated in the brain, and rapid eye movement sleep (REMS) is enhanced compared with wild-type mice. We hypothesized that such sleep impairment at adulthood results from excessive serotonergic tone during early life. Thus, we assessed whether neonatal treatment with drugs capable of limiting the impact of 5-HT on the brain could normalize sleep patterns in 5-HTT-/- mutants. We found that treatments initiated at postnatal day 5 and continued for 2 weeks with the 5-HT synthesis inhibitor para-chlorophenylalanine, or for 4 weeks with the 5-HT(1A) receptor (5-HT(1A)R) antagonist N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridinyl) cyclohexane carboxamide (WAY 100635), induced total or partial recovery of REMS, respectively, in 5-HTT-/- mutants. Early life treatment with WAY 100635 also reversed the depression-like behavior otherwise observed in these mutants. Possible adaptive changes in 5-HT(1A)R after neonatal treatment with WAY 100635 were investigated by measuring 5-HT(1A) binding sites and 5-HT(1A) mRNA in various REMS- and/or depression-related brain areas, as well as 5-HT(1A)R-mediated hypothermia and inhibition of neuronal firing in the dorsal raphe nucleus. None of these characteristics were modified in parallel with REMS recovery, suggesting that 5-HT(1A)Rs involved in wild-type phenotype rescue in 5-HTT-/- mutants are located in other brain areas or in 5-HT(1A)R-unrelated circuits where they could be transiently expressed during development. The reversal of sleep alterations and depression-like behavior after early life blockade of 5-HT(1A)R in 5-HTT-/- mutants might open new perspectives regarding preventive care of sleep and mood disorders resulting from serotonin transporter impairments during development.
Figures


References
-
- Adrien J (2002). Neurobiological bases for the relation between sleep and depression. Sleep Med Rev 6:351–352. - PubMed
-
- Alexandre C, Popa D, Bouali S, Léna C, Hamon M, Adrien J (2003). Inhibition of serotonin synthesis during development restores normal REM sleep level in 5-HT transporter knock-out mice. Sleep 26:[Abstr Suppl], 0071A.
-
- Amici R, Sanford LD, Kearney K, McInerney B, Ross RJ, Horner RL, Morrison AR (2004). A serotonergic (5-HT2) receptor mechanism in the laterodorsal tegmental nucleus participates in regulating the pattern of rapid-eye-movement sleep occurrence in the rat. Brain Res 996:9–18. - PubMed
-
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA (2004). Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science 306:879–881. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
