HPV16-E6 associated hTERT promoter acetylation is E6AP dependent, increased in later passage cells and enhanced by loss of p300
- PMID: 16708385
- PMCID: PMC2223064
- DOI: 10.1002/ijc.22064
HPV16-E6 associated hTERT promoter acetylation is E6AP dependent, increased in later passage cells and enhanced by loss of p300
Abstract
The E6 oncoprotein from high-risk HPV types activates human telomerase reverse transcriptase (hTERT) transcription in human keratinocytes. Studies on how E6 regulates hTERT have implicated E-box or X-box elements in the hTERT promoter (Veldman et al., Proc Natl Acad Sci USA 2003;100:8211-14; Oh et al., J Virol 2001;75:5559-66; Gewin et al., Genes Dev 2004;18:2269-82), but the mechanism of activation by E6 is still controversial and not well defined. Here, we demonstrate that induction of both hTERT expression and telomerase activity by HPV-16 E6 in early passage keratinocytes is associated with acetylation of histone H3 at the hTERT promoter, is dependent on the E6 associated protein (E6AP) and is not exclusively reliant on E-box or X-box elements. Further increases in histone acetylation of the hTERT promoter and hTERT transcriptional activity in E6 expressing cells that had been passaged extensively in culture were found to occur only with the endogenous promoter and not with an exogenously introduced hTERT promoter construct. Telomerase activity at both early and late passages, however, was dependent on E6AP expression, implying a continued reliance on E6 function for telomerase activity. Our results demonstrate that E6 induces hTERT promoter acetylation, but that further increases in telomerase activity and histone acetylation in later passage E6 expressing cells are independent of E6 activation of the core hTERT promoter. We also provide evidence that the transcription factor p300 is a potential repressor of telomerase activation and histone acetylation in the context of E6 expression. These studies give insight into how immortalization by HPV results in upregulation of hTERT and furthers our understanding of how telomerase is activated during the process of malignant transformation.
Copyright 2006 Wiley-Liss, Inc.
Figures

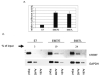


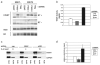
Similar articles
-
NFX1 interacts with mSin3A/histone deacetylase to repress hTERT transcription in keratinocytes.Mol Cell Biol. 2008 Aug;28(15):4819-28. doi: 10.1128/MCB.01969-07. Epub 2008 May 27. Mol Cell Biol. 2008. PMID: 18505829 Free PMC article.
-
The E6AP ubiquitin ligase is required for transactivation of the hTERT promoter by the human papillomavirus E6 oncoprotein.J Biol Chem. 2005 Mar 18;280(11):10807-16. doi: 10.1074/jbc.M410343200. Epub 2005 Jan 17. J Biol Chem. 2005. PMID: 15655249
-
Binding of human papillomavirus type 16 E6 to E6AP is not required for activation of hTERT.J Virol. 2008 Jan;82(1):71-6. doi: 10.1128/JVI.01776-07. Epub 2007 Oct 17. J Virol. 2008. PMID: 17942561 Free PMC article.
-
Activation of telomerase by HPVs.Virus Res. 2017 Mar 2;231:50-55. doi: 10.1016/j.virusres.2016.11.003. Epub 2016 Nov 15. Virus Res. 2017. PMID: 27863966 Review.
-
Transcriptional regulation of the telomerase hTERT gene as a target for cellular and viral oncogenic mechanisms.Carcinogenesis. 2003 Jul;24(7):1167-76. doi: 10.1093/carcin/bgg085. Epub 2003 May 22. Carcinogenesis. 2003. PMID: 12807729 Review.
Cited by
-
Long non-coding RNA FAM83H-AS1 is regulated by human papillomavirus 16 E6 independently of p53 in cervical cancer cells.Sci Rep. 2019 Mar 6;9(1):3662. doi: 10.1038/s41598-019-40094-8. Sci Rep. 2019. PMID: 30842470 Free PMC article.
-
NFX1 interacts with mSin3A/histone deacetylase to repress hTERT transcription in keratinocytes.Mol Cell Biol. 2008 Aug;28(15):4819-28. doi: 10.1128/MCB.01969-07. Epub 2008 May 27. Mol Cell Biol. 2008. PMID: 18505829 Free PMC article.
-
Attenuated Recombinant Influenza A Virus Expressing HPV16 E6 and E7 as a Novel Therapeutic Vaccine Approach.PLoS One. 2015 Sep 18;10(9):e0138722. doi: 10.1371/journal.pone.0138722. eCollection 2015. PLoS One. 2015. PMID: 26381401 Free PMC article.
-
Telomerase Induction in HPV Infection and Oncogenesis.Viruses. 2017 Jul 10;9(7):180. doi: 10.3390/v9070180. Viruses. 2017. PMID: 28698524 Free PMC article. Review.
-
Direct HPV E6/Myc interactions induce histone modifications, Pol II phosphorylation, and hTERT promoter activation.Oncotarget. 2017 Oct 25;8(56):96323-96339. doi: 10.18632/oncotarget.22036. eCollection 2017 Nov 10. Oncotarget. 2017. PMID: 29221209 Free PMC article.
References
-
- Jones DL, Thompson DA, Munger K. Destabilization of the RB tumor suppressor protein and stabilization of p53 contribute to HPV type 16 E7-induced apoptosis. Virology. 1997;239:97–107. - PubMed
-
- Elbel M, Carl S, Spaderna S, Iftner T. A comparative analysis of the interactions of the E6 proteins from cutaneous and genital papillomaviruses with p53 and E6AP in correlation to their transforming potential. Virology. 1997;239:132–49. - PubMed
-
- Klingelhutz AJ, Foster SA, McDougall JK. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature. 1996;380:79–82. - PubMed
-
- Baege AC, Berger A, Schlegel R, Veldman T. Cervical epithelial cells transduced with the papillomavirus E6/E7 oncogenes maintain stable levels of oncoprotein expression but exhibit progressive, major increases in hTERT gene expression and telomerase activity. Am J Pathol. 2002;160:1251–7. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

