AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues
- PMID: 16709629
- PMCID: PMC1817809
- DOI: 10.1113/jphysiol.2006.113217
AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues
Abstract
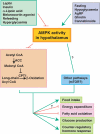
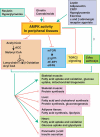
The evolutionarily conserved serine/threonine kinase, AMP-activated protein kinase (AMPK), functions as a cellular fuel gauge that regulates metabolic pathways in glucose and fatty acid metabolism and protein synthesis. Recent data strongly implicate the AMPK-acetyl CoA carboxylase (ACC)-malonyl CoA pathway in the hypothalamus in the regulation of food intake, body weight and hepatic glucose production. Furthermore, data indicate that AMPK is a mediator of the effects of adipocyte-derived and gut-derived hormones and peptides on fatty acid oxidation and glucose uptake in peripheral tissues. Studies are now elucidating the potential role of kinases upstream of AMPK in these metabolic effects. In addition, recently, several novel downstream effectors of AMPK have been identified. The AMPK pathway in the hypothalamus and peripheral tissues coordinately integrates inputs from multiple hormones, peptides and nutrients to maintain energy homeostasis.
Figures
References
-
- Anderson KA, Means RL, Huang QH, Kemp BE, Goldstein EG, Selbert MA, Edelman AM, Fremeau RT, Means AR. Components of a calmodulin-dependent protein kinase cascade. J Biol Chem. 1998;273:31880–31889. - PubMed
-
- Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem. 2004;279:12005–12008. - PubMed
-
- Banerjee RR, Rangwala SM, Shapiro JS, Rich AS, Rhoades B, Qi Y, et al. Regulation of fasted blood glucose by resistin. Science. 2004;303:1195–1198. - PubMed
-
- Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMPK-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277:23977–23980. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

