'Simplification' of responses of complex cells in cat striate cortex: suppressive surrounds and 'feedback' inactivation
- PMID: 16709635
- PMCID: PMC1817736
- DOI: 10.1113/jphysiol.2006.110320
'Simplification' of responses of complex cells in cat striate cortex: suppressive surrounds and 'feedback' inactivation
Abstract
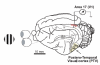
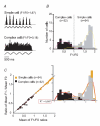
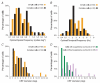
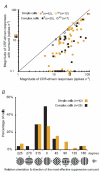
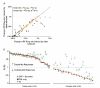
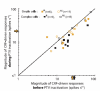
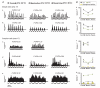
In mammalian striate cortex (V1), two distinct functional classes of neurones, the so-called simple and complex cells, are routinely distinguished. They can be quantitatively differentiated from each other on the basis of the ratio between the phase-variant (F1) component and the mean firing rate (F0) of spike responses to luminance-modulated sinusoidal gratings (simple, F1/F0 > 1; complex, F1/F0 < 1). We investigated how recurrent cortico-cortical connections affect the spatial phase-variance of responses of V1 cells in the cat. F1/F0 ratios of the responses to optimally oriented drifting sine-wave gratings covering the classical receptive field (CRF) of single V1 cells were compared to those of: (1) responses to gratings covering the CRFs combined with gratings of different orientations presented to the 'silent' surrounds; and (2) responses to CRF stimulation during reversible inactivation of postero-temporal visual (PTV) cortex. For complex cells, the relative strength of the silent surround suppression on CRF-driven responses was positively correlated with the extent of increases in F1/F0 ratios. Inactivation of PTV cortex increased F1/F0 ratios of CRF-driven responses of complex cells only. Overall, activation of suppressive surrounds or inactivation of PTV 'converted' substantial proportions (50 and 30%, respectively) of complex cells into simple-like cells (F1/F0 > 1). Thus, the simple-complex distinction depends, at least partly, on information coming from the silent surrounds and/or feedback from 'higher-order' cortices. These results support the idea that simple and complex cells belong to the same basic cortical circuit and the spatial phase-variance of their responses depends on the relative strength of different synaptic inputs.
Figures



References
-
- Akasaki T, Sato H, Yoshimura Y, Ozeki H, Shimegi S. Suppressive effects of receptive field surround on neuronal activity in the cat primary visual cortex. Neurosci Res. 2002;43:207–220. - PubMed
-
- Bair W. Visual receptive field organization. Curr Opin Neurobiol. 2005;15:1–6. - PubMed
-
- Bardy C, Huang JY, FitzGibbon T, Wang C, Dreher B. Suppressive surrounds ‘simplify’ complex cells in cat's primary visual cortex. Proc Austral Neurosci Soc. 2006;17:39.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

