T-tubule disorganization and reduced synchrony of Ca2+ release in murine cardiomyocytes following myocardial infarction
- PMID: 16709642
- PMCID: PMC1817777
- DOI: 10.1113/jphysiol.2006.107227
T-tubule disorganization and reduced synchrony of Ca2+ release in murine cardiomyocytes following myocardial infarction
Abstract
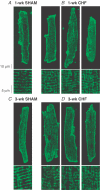
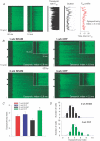
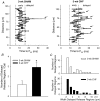
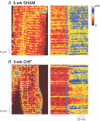
In cardiac myocytes, initiation of excitation-contraction coupling is highly localized near the T-tubule network. Myocytes with a dense T-tubule network exhibit rapid and homogeneous sarcoplasmic reticulum (SR) Ca(2+) release throughout the cell. We examined whether progressive changes in T-tubule organization and Ca(2+) release synchrony occur in a murine model of congestive heart failure (CHF). Myocardial infarction (MI) was induced by ligation of the left coronary artery, and CHF was diagnosed by echocardiography (left atrial diameter >2.0 mm). CHF mice were killed at 1 or 3 weeks following MI (1-week CHF, 3-week CHF) and cardiomyocytes were isolated from viable regions of the septum, excluding the MI border zone. Septal myocytes from SHAM-operated mice served as controls. T-tubules were visualized by confocal microscopy in cells stained with di-8-ANEPPS. SHAM cells exhibited a regular striated T-tubule pattern. However, 1-week CHF cells showed slightly disorganized T-tubule structure, and more profound disorganization occurred in 3-week CHF with irregular gaps between adjacent T-tubules. Line-scan images of Ca(2+) transients (fluo-4 AM, 1 Hz) showed that regions of delayed Ca(2+) release occurred at these gaps. Three-week CHF cells exhibited an increased number of delayed release regions, and increased overall dyssynchrony of Ca(2+) release. A common pattern of Ca(2+) release in 3-week CHF was maintained between consecutive transients, and was not altered by forskolin application. Thus, progressive T-tubule disorganization during CHF promotes dyssynchrony of SR Ca(2+) release which may contribute to the slowing of SR Ca(2+) release in this condition.
Figures



Comment in
-
T-tubule trouble.J Physiol. 2006 Jul 15;574(Pt 2):330. doi: 10.1113/jphysiol.2006.113803. Epub 2006 May 25. J Physiol. 2006. PMID: 16728445 Free PMC article. No abstract available.
References
-
- Balijepalli RC, Lokuta AJ, Maertz NA, Buck JM, Haworth RA, Valdivia HH, Kamp TJ. Depletion of T-tubules and specific subcellular changes in sarcolemmal proteins in tachycardia-induced heart failure. Cardiovasc Res. 2003;59:67–77. - PubMed
-
- Berlin JR. Spatiotemporal changes of Ca2+ during electrically evoked contractions in atrial and ventricular cells. Am J Physiol. 1995;269:H1165–H1170. - PubMed
-
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. - PubMed
-
- Beuckelmann DJ, Erdmann E. Ca2+-currents and intracellular [Ca2+]i-transients in single ventricular myocytes isolated from terminally failing human myocardium. Basic Res Cardiol. 1992;87(Suppl. 1):235–243. - PubMed
-
- Brette F, Rodriguez P, Komukai K, Colyer J, Orchard CH. Beta-adrenergic stimulation restores the Ca2+ transient of ventricular myocytes lacking T-tubules. J Mol Cell Cardiol. 2004;36:265–275. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

