Contractile properties of EDL and soleus muscles of myostatin-deficient mice
- PMID: 16709649
- PMCID: PMC4088255
- DOI: 10.1152/japplphysiol.00126.2006
Contractile properties of EDL and soleus muscles of myostatin-deficient mice
Abstract
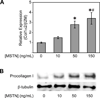
Myostatin is a negative regulator of muscle mass. The impact of myostatin deficiency on the contractile properties of healthy muscles has not been determined. We hypothesized that myostatin deficiency would increase the maximum tetanic force (P(o)), but decrease the specific P(o) (sP(o)) of muscles and increase the susceptibility to contraction-induced injury. The in vitro contractile properties of extensor digitorum longus (EDL) and soleus muscles from wild-type (MSTN(+/+)), heterozygous-null (MSTN(+/-)), and homozygous-null (MSTN(-/-)) adult male mice were determined. For EDL muscles, the P(o) of both MSTN(+/-) and MSTN(-/-) mice were greater than the P(o) of MSTN(+/+) mice. For soleus muscles, the P(o) of MSTN(-/-) mice was greater than that of MSTN(+/+) mice. The sP(o) of EDL muscles of MSTN(-/-) mice was less than that of MSTN(+/+) mice. For soleus muscles, however, no difference in sP(o) was observed. Following two lengthening contractions, EDL muscles from MSTN(-/-) mice had a greater force deficit than that of MSTN(+/+) or MSTN(+/-) mice, whereas no differences were observed for the force deficits of soleus muscles. Myostatin-deficient EDL muscles had less hydroxyproline, and myostatin directly increased type I collagen mRNA expression and protein content. The difference in the response of EDL and soleus muscles to myostatin may arise from differences in the levels of a myostatin receptor, activin type IIB. Compared with the soleus, the amount of activin type IIB receptor was approximately twofold greater in EDL muscles. The results support a significant role for myostatin not only in the mass of muscles but also in the contractility and the composition of the extracellular matrix of muscles.
Conflict of interest statement
The authors report no conflict of interest.
Figures



References
-
- Allen RE, Temm-Grove CJ, Sheehan SM, Rice G. Skeletal muscle satellite cell cultures. Methods Cell Biol. 1997;52:155–176. - PubMed
-
- Amthor H, Nicholas G, McKinnell I, Kemp CF, Sharma M, Kambadur R, Patel K. Follistatin complexes Myostatin and antagonises Myostatin-mediated inhibition of myogenesis. Dev Biol. 2004;270:19–30. - PubMed
-
- Bernasconi P, Torchiana E, Confalonieri P, Brugnoni R, Barresi R, Mora M, Cornelio F, Morandi L, Mantegazza R. Expression of transforming growth factor-beta 1 in dystrophic patient muscles correlates with fibrosis. Pathogenetic role of a fibrogenic cytokine. J Clin Invest. 1995;96:1137–1144. - PMC - PubMed
-
- Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS, Khurana TS. Functional improvement of dystrophic muscle by myostatin blockade. Nature. 2002;420:418–421. - PubMed
-
- Bogdanovich S, Perkins KJ, Krag TO, Whittemore LA, Khurana TS. Myostatin propeptide-mediated amelioration of dystrophic pathophysiology. Faseb J. 2005;19:543–549. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

