A novel fluorescent probe that is brain permeable and selectively binds to myelin
- PMID: 16709728
- PMCID: PMC3150784
- DOI: 10.1369/jhc.5A6901.2006
A novel fluorescent probe that is brain permeable and selectively binds to myelin
Abstract
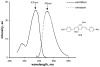
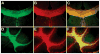
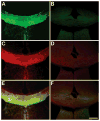
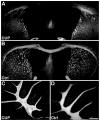
Myelin is a multilayered glial cell membrane that forms segmented sheaths around large-caliber axons of both the central nervous system (CNS) and peripheral nervous system (PNS). Myelin covering insures rapid and efficient transmission of nerve impulses. Direct visual assessment of local changes of myelin content in vivo could greatly facilitate diagnosis and therapeutic treatments of myelin-related diseases. Current histologic probes for the visualization of myelin are based on antibodies or charged histochemical reagents that do not enter the brain. We have developed a series of chemical compounds including (E,E)-1,4-bis(4'-aminostyryl)-2-dimethoxy-benzene termed BDB and the subject of this report, which readily penetrates the blood-brain barrier and selectively binds to the myelin sheath in brain. BDB selectively stains intact myelinated regions in wild-type mouse brain, which allows for delineation of cuprizone-induced demyelinating lesions in mouse brain. BDB can be injected IV into the brain and selectively detect demyelinating lesions in cuprizone-treated mice in situ. These studies justified further investigation of BDB as a potential myelin-imaging probe to monitor myelin pathology in vivo.
Figures



References
-
- Bancroft J, Gamble M. Theory and Practice of Histological Techniques. 5. London: Churchill-Livingstone; 2002.
-
- Berube GR, Powers MM, Clark G. Iron hematoxylin chelates. I. The Weil staining bath. Stain Technol. 1965;40:53–62. - PubMed
-
- Clark SL, Ward JW. A variation of the Pal-Weigert method for staining myelin sheaths. Stain Technol. 1934;54:13–16.
-
- Cook H. Manual of Histological Demonstration Methods. 5. London: Butterworth; 1974.
-
- Horton JC, Hocking DR. Myelin patterns in V1 and V2 of normal and monocularly enucleated monkeys. Cereb Cortex. 1997;7:166–177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

