NR2B tyrosine phosphorylation modulates fear learning as well as amygdaloid synaptic plasticity
- PMID: 16710293
- PMCID: PMC1500840
- DOI: 10.1038/sj.emboj.7601156
NR2B tyrosine phosphorylation modulates fear learning as well as amygdaloid synaptic plasticity
Abstract
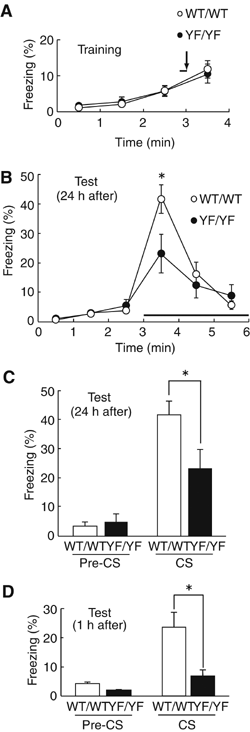
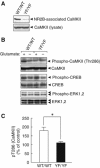
Phosphorylation of neural proteins in response to a diverse array of external stimuli is one of the main mechanisms underlying dynamic changes in neural circuitry. The NR2B subunit of the NMDA receptor is tyrosine-phosphorylated in the brain, with Tyr-1472 its major phosphorylation site. Here, we generate mice with a knockin mutation of the Tyr-1472 site to phenylalanine (Y1472F) and show that Tyr-1472 phosphorylation is essential for fear learning and amygdaloid synaptic plasticity. The knockin mice show impaired fear-related learning and reduced amygdaloid long-term potentiation. NMDA receptor-mediated CaMKII signaling is impaired in YF/YF mice. Electron microscopic analyses reveal that the Y1472F mutant of the NR2B subunit shows improper localization at synapses in the amygdala. We thus identify Tyr-1472 phosphorylation as a key mediator of fear learning and amygdaloid synaptic plasticity.
Figures




References
-
- Barria A, Malinow R (2005) NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron 48: 289–301 - PubMed
-
- Bayer KU, Koninck PD, Leonard AS, Hell JW, Schulman H (2001) Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature 411: 801–805 - PubMed
-
- Blanpied TA, Scott DB, Ehlers MD (2002) Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines. Neuron 36: 435–449 - PubMed
-
- Bliss TVP, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31–39 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

