Legionella pneumophila exploits PI(4)P to anchor secreted effector proteins to the replicative vacuole
- PMID: 16710455
- PMCID: PMC1463015
- DOI: 10.1371/journal.ppat.0020046
Legionella pneumophila exploits PI(4)P to anchor secreted effector proteins to the replicative vacuole
Abstract
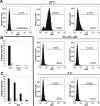
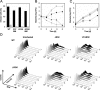
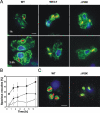
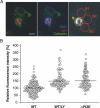
The causative agent of Legionnaires' disease, Legionella pneumophila, employs the intracellular multiplication (Icm)/defective organelle trafficking (Dot) type IV secretion system (T4SS) to upregulate phagocytosis and to establish a replicative vacuole in amoebae and macrophages. Legionella-containing vacuoles (LCVs) do not fuse with endosomes but recruit early secretory vesicles. Here we analyze the role of host cell phosphoinositide (PI) metabolism during uptake and intracellular replication of L. pneumophila. Genetic and pharmacological evidence suggests that class I phosphatidylinositol(3) kinases (PI3Ks) are dispensable for phagocytosis of wild-type L. pneumophila but inhibit intracellular replication of the bacteria and participate in the modulation of the LCV. Uptake and degradation of an icmT mutant strain lacking a functional Icm/Dot transporter was promoted by PI3Ks. We identified Icm/Dot-secreted proteins which specifically bind to phosphatidylinositol(4) phosphate (PI(4)P) in vitro and preferentially localize to LCVs in the absence of functional PI3Ks. PI(4)P was found to be present on LCVs using as a probe either an antibody against PI(4)P or the PH domain of the PI(4)P-binding protein FAPP1 (phosphatidylinositol(4) phosphate adaptor protein-1). Moreover, the presence of PI(4)P on LCVs required a functional Icm/Dot T4SS. Our results indicate that L. pneumophila modulates host cell PI metabolism and exploits the Golgi lipid second messenger PI(4)P to anchor secreted effector proteins to the LCV.
Conflict of interest statement
Figures



References
-
- Fields BS. The molecular ecology of Legionellae . Trends Microbiol. 1996;4:286–290. - PubMed
-
- McDade JE, Shepard CC, Fraser DW, Tsai TR, Redus MA, et al. Legionnaires' disease: Isolation of a bacterium and demonstration of its role in other respiratory disease. N Engl J Med. 1977;297:1197–1203. - PubMed
-
- Kagan JC, Roy CR. Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nat Cell Biol. 2002;4:945–954. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

