Site-specific 3D imaging of cells and tissues with a dual beam microscope
- PMID: 16713294
- PMCID: PMC1647295
- DOI: 10.1016/j.jsb.2006.03.006
Site-specific 3D imaging of cells and tissues with a dual beam microscope
Abstract
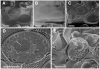
Current approaches to 3D imaging at subcellular resolution using confocal microscopy and electron tomography, while powerful, are limited to relatively thin and transparent specimens. Here we report on the use of a new generation of dual beam electron microscopes capable of site-specific imaging of the interior of cellular and tissue specimens at spatial resolutions about an order of magnitude better than those currently achieved with optical microscopy. The principle of imaging is based on using a focused ion beam to create a cut at a designated site in the specimen, followed by viewing the newly generated surface with a scanning electron beam. Iteration of these two steps several times thus results in the generation of a series of surface maps of the specimen at regularly spaced intervals, which can be converted into a three-dimensional map of the specimen. We have explored the potential of this sequential "slice-and-view" strategy for site-specific 3D imaging of frozen yeast cells and tumor tissue, and establish that this approach can identify the locations of intracellular features such as the 100 nm-wide yeast nuclear pore complex. We also show that 200 nm thick sections can be generated in situ by "milling" of resin-embedded specimens using the ion beam, providing a valuable alternative to manual sectioning of cells and tissues using an ultramicrotome. Our results demonstrate that dual beam imaging is a powerful new tool for cellular and subcellular imaging in 3D for both basic biomedical and clinical applications.
Figures




References
-
- Ballerini M, Milani M, Batani M, Squadrini F. Focused ion beam techniques for the analysis of biological samples: a revolution in ultramicroscopy? Proc. SPIE. 2001;4261:92–104.
-
- Barnard DP, Turner JN, Frank J, McEwen BF. A 360 degrees single-axis tilt stage for the high-voltage electron microscope. J. Microsc. 1992;167(Pt 1):39–48. - PubMed
-
- Bauer C, Herzog V, Bauer MF. Improved technique for electron microscope visualization of yeast membrane structure. Microsc. Microanal. 2001;7:530–534. - PubMed
-
- Drobne D, Milani M, Zrimec A, Zrimec MB, Tatti F, Draslar K. Focused ion beam/scanning electron microscopy studies of Porcellio scaber (Isopoda, Crustacea) digestive gland epithelium cells. Scanning. 2005;27:30–34. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

