Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8
- PMID: 16713360
- PMCID: PMC1564441
- DOI: 10.1016/j.ymthe.2006.03.014
Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8
Abstract
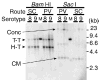
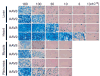
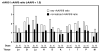
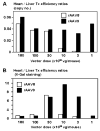
It has been recently shown that recombinant adeno-associated virus serotype 8 (rAAV8) is a robust alternative serotype vector that overcomes many of the limitations of rAAV2 and transduces various tissues efficiently and globally through systemic vector administration. AAV9 is a serotype newly isolated from human tissues, but our knowledge of the biology of rAAV9 in vivo is currently limited. Here, we demonstrate by a series of comprehensive side-by-side experiments with rAAV8 and 9 vectors delivered via different routes or at various doses in mice that rAAV9 vectors share the robustness of rAAV8, i.e., (1) very high liver transduction efficiency irrespective of whether vectors are administered intravascularly or extravascularly and (2) substantial transduction in the heart, skeletal muscle, and pancreas by peripheral vein injection. Importantly, rAAV9 transduced myocardium 5- to 10-fold higher than rAAV8, resulting in over 80% cardiomyocyte transduction following tail vein injection of as low as 1.0 x 10(11) particles per mouse. Thus rAAV9, as well as rAAV8, is a robust vector for gene therapy applications and rAAV9 is superior to rAAV8 specifically for cardiac gene delivery by systemic vector administration.
Figures

References
-
- Muzyczka N, Warrington KH., Jr Custom adeno-associated virus capsids: the next generation of recombinant vectors with novel tropism. Hum Gene Ther. 2005;16:408–416. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

