Membrane lateral diffusion and capture of CFTR within transient confinement zones
- PMID: 16714353
- PMCID: PMC1563763
- DOI: 10.1529/biophysj.106.084830
Membrane lateral diffusion and capture of CFTR within transient confinement zones
Abstract
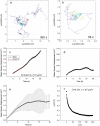
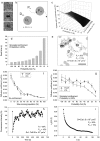
The cystic fibrosis transmembrane conductance regulator (CFTR) channel interacts with scaffolding and other proteins that are expected to restrict its lateral movement, yet previous studies have reported predominantly free diffusion. We examined the lateral mobility of CFTR channels on live baby hamster kidney cells using three complementary methods. Channels bearing an extracellular biotinylation target sequence were labeled with streptavidin conjugated with fluorescent dyes (Alexa Fluor 488 or 568) or quantum dots (qDot605). Fluorescence recovery after photobleaching and image correlation spectroscopy of the dye-labeled channels revealed a significant immobile population ( approximately 50%), which was confirmed by direct single particle tracking (SPT) of qDot605-labeled CFTR. Adding 10 histidine residues at the C-terminus of CFTR to mask the postsynaptic density 95, Discs large, ZO-1 (PDZ) binding motif abolished its association with EBP50/NHERF1, reduced the immobile fraction, and increased mobility. Other interactions that are not normally detected on this timescale became apparent when binding of PDZ domain proteins was disrupted. SPT revealed that CFTR(His-10) channels diffuse randomly, become immobilized for periods lasting up to 1 min, and in some instances are recaptured at the same location. The impact of transient confinement on the measured diffusion using the three fluorescence techniques were assessed using computer simulations of the biological experiments. Finally, the impact of endosomal CFTR on mobility measurements was assessed by fluorescence correlation spectroscopy. These results reveal unexpected features of CFTR dynamics which may influence its ion channel activity.
Figures




References
-
- Riordan, J. R., J. M. Rommens, B. Kerem, N. Alon, R. Rozmahel, Z. Grzelczak, J. Zielenski, S. Lok, N. Plaversusic, and J. L. Chou. 1989. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 245:1066–1073. - PubMed
-
- Voltz, J. W., E. J. Weinman, and S. Shenolikar. 2001. Expanding the role of NHERF, a PDZ-domain containing protein adapter, to growth regulation. Oncogene. 20:6309–6314. - PubMed
-
- Li, C., K. Roy, K. Dandridge, and A. P. Naren. 2004. Molecular assembly of cystic fibrosis transmembrane conductance regulator in plasma membrane. J. Biol. Chem. 279:24673–24684. - PubMed
-
- Milewski, M. I., J. E. Mickle, J. K. Forrest, D. M. Stafford, B. D. Moyer, J. Cheng, W. B. Guggino, B. A. Stanton, and G. R. Cutting. 2001. A PDZ-binding motif is essential but not sufficient to localize the C terminus of CFTR to the apical membrane. J. Cell Sci. 114:719–726. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

