The tale beyond the tail: histone core domain modifications and the regulation of chromatin structure
- PMID: 16714444
- PMCID: PMC1464108
- DOI: 10.1093/nar/gkl338
The tale beyond the tail: histone core domain modifications and the regulation of chromatin structure
Abstract
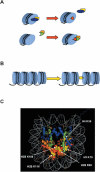
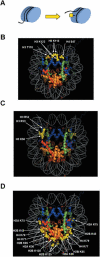
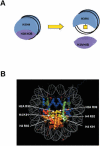
Histone post-translational modifications occur, not only in the N-terminal tail domains, but also in the core domains. While modifications in the N-terminal tail function largely through the regulation of the binding of non-histone proteins to chromatin, based on their location in the nucleosome, core domain modifications may also function through distinct mechanisms involving structural alterations to the nucleosome. This article reviews the recent developments in regards to these novel histone modifications and discusses their important role in the regulation of chromatin structure.
Figures
References
-
- Murray K. The occurrence of epsilon-N-methyl lysine in histones. Biochemistry. 1964;127:10–15. - PubMed
-
- DeLange R.J., Fambrough D.M., Smith E.L., Bonner J. Calf and pea histone IV. I. Amino acid compositions and the identical COOH-terminal 19-residue sequence. J. Biol. Chem. 1968;243:5906–5913. - PubMed
-
- Gershey E.L., Vidali G., Allfrey V.G. Chemical studies of histone acetylation. The occurrence of epsilon-N-acetyllysine in the f2a1 histone. J. Biol. Chem. 1968;243:5018–5022. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

