Salmonella enterica serovar Typhimurium requires the Lpf, Pef, and Tafi fimbriae for biofilm formation on HEp-2 tissue culture cells and chicken intestinal epithelium
- PMID: 16714543
- PMCID: PMC1479237
- DOI: 10.1128/IAI.01428-05
Salmonella enterica serovar Typhimurium requires the Lpf, Pef, and Tafi fimbriae for biofilm formation on HEp-2 tissue culture cells and chicken intestinal epithelium
Abstract
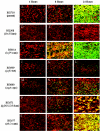
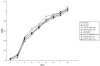
Recent work has demonstrated that Salmonella enterica serovar Typhimurium forms biofilms on HEp-2 tissue culture cells in a type 1 fimbria-dependent manner. To investigate how biofilm growth of HEp-2 tissue culture cells affects gene expression in Salmonella, we compared global gene expression during planktonic growth and biofilm growth. Microarray results indicated that the transcription of approximately 100 genes was substantially altered by growth in a biofilm. These genes encode proteins with a wide range of functions, including antibiotic resistance, central metabolism, conjugation, intracellular survival, membrane transport, regulation, and fimbrial biosynthesis. The identification of five fimbrial gene clusters was of particular interest, as we have demonstrated that type 1 fimbriae are required for biofilm formation on HEp-2 cells and murine intestinal epithelium. Mutations in each of these fimbriae were constructed in S. enterica serovar Typhimurium strain BJ2710, and the mutants were found to have various biofilm phenotypes on plastic, HEp-2 cells, and chicken intestinal tissue. The pef and csg mutants were defective for biofilm formation on each of the three surfaces tested, while the lpf mutant exhibited a complete loss of the ability to form a biofilm on chicken intestinal tissue but only an intermediate loss of the ability to form a biofilm on tissue culture cells and plastic surfaces. The bcf mutant displayed increased biofilm formation on both HEp-2 cells and chicken intestinal epithelium, while the sth mutant had no detectable biofilm defects. In all instances, the mutants could be restored to a wild-type phenotype by a plasmid carrying the functional genes. This is the first work to identify the genomic responses of Salmonella to biofilm formation on host cells, and this work highlights the importance of fimbriae in adhering to and adapting to a eukaryotic cell surface. An understanding of these interactions is likely to provide new insights for intervention strategies in Salmonella colonization and infection.
Figures
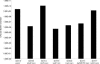



References
-
- Austin, J. W., G. Sanders, W. W. Kay, and S. K. Collinson. 1998. Thin aggregative fimbriae enhance Salmonella enteritidis biofilm formation. FEMS Microbiol. Lett. 162:295-301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

