Differential requirements for soluble and transmembrane tumor necrosis factor in the immunological control of primary and secondary Listeria monocytogenes infection
- PMID: 16714545
- PMCID: PMC1479262
- DOI: 10.1128/IAI.02004-05
Differential requirements for soluble and transmembrane tumor necrosis factor in the immunological control of primary and secondary Listeria monocytogenes infection
Abstract
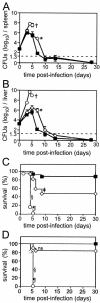
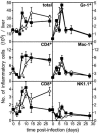
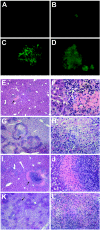
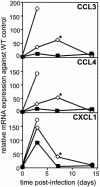
The relative contributions of transmembrane tumor necrosis factor (memTNF) and soluble tumor necrosis factor (solTNF) in innate and adaptive immunity are poorly defined. We examined the capacities of wild-type (WT) mice, TNF-/- mice, and memTNF mice, which express only transmembrane TNF, to control primary and secondary Listeria monocytogenes infections. Soluble TNF was not required for induction or maintenance of protective immunity against a low-dose (200-CFU) Listeria infection. In contrast to TNF-/- mice, both WT and memTNF mice cleared the bacilli within 10 days and were fully protected against rechallenge with a lethal infective dose. Furthermore, T cells transferred from immune mice, but not from naïve, WT, and memTNF mice, protected TNF-/- recipients against an otherwise lethal infection. By contrast, infection with a higher dose of Listeria (2,000 CFU) clearly demonstrated that solTNF is required to coordinate an optimal protective inflammatory response. memTNF mice were more susceptible to a high-dose infection, and they exhibited delayed bacterial clearance, increased inflammation, and necrosis in the liver that resulted in 55% mortality. The dysregulated inflammation was accompanied by prolonged elevated expression of mRNAs for several chemokines as well as the macrophage effector molecules inducible nitric oxide synthase and LRG-47 in the livers of memTNF mice but not in the livers of WT mice. These data demonstrated that memTNF is sufficient for establishing protective immunity against a primary low-dose Listeria infection but that solTNF is required for optimal control of cellular inflammation and resistance to a primary high-dose infection. By contrast, memTNF alone is sufficient for resolution of a secondary, high-dose infection and for the transfer of protective immunity with memory T cells.
Figures




References
-
- Bouwer, H. G., R. A. Barry, and D. J. Hinrichs. 1997. Acquired immunity to an intracellular pathogen: immunologic recognition of L. monocytogenes-infected cells. Immunol. Rev. 158:137-146. - PubMed
-
- Bowie, V. L., K. A. Snella, A. S. Gopalachar, and P. Bharadwaj. 2004. Listeria meningitis associated with infliximab. Ann. Pharmacother. 38:58-61. - PubMed
-
- Collazo, C. M., G. S. Yap, G. D. Sempowski, K. C. Lusby, L. Tessarollo, G. F. Woude, A. Sher, and G. A. Taylor. 2001. Inactivation of LRG-47 and IRG-47 reveals a family of interferon gamma-inducible genes with essential, pathogen-specific roles in resistance to infection. J. Exp. Med. 194:181-188. - PMC - PubMed
-
- Czuprynski, C. J., J. F. Brown, N. Maroushek, R. D. Wagner, and H. Steinberg. 1994. Administration of anti-granulocyte mAb RB6-8C5 impairs the resistance of mice to Listeria monocytogenes infection. J. Immunol. 152:1836-1846. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

