Attenuation and persistence of and ability to induce protective immunity to a Staphylococcus aureus aroA mutant in mice
- PMID: 16714581
- PMCID: PMC1479249
- DOI: 10.1128/IAI.01507-05
Attenuation and persistence of and ability to induce protective immunity to a Staphylococcus aureus aroA mutant in mice
Abstract
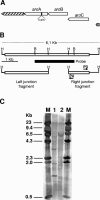
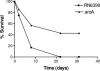
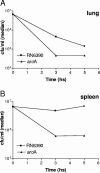
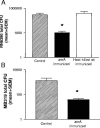
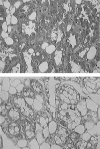
Staphylococcus aureus is the most important etiological agent of bovine mastitis, a disease that causes significant economic losses to the dairy industry. Several vaccines to prevent the disease have been tested, with limited success. The aim of this study was to obtain a suitable attenuated aro mutant of S. aureus by transposon mutagenesis and to demonstrate its efficacy as a live vaccine to induce protective immunity in a murine model of intramammary infection. To do this, we transformed S. aureus RN6390 with plasmid pTV1ts carrying Tn917. After screening of 3,493 erythromycin-resistant colonies, one mutant incapable of growing on plates lacking phenylalanine, tryptophan, and tyrosine was isolated and characterized. Molecular characterization of the mutant showed that the affected gene was aroA and that the insertion occurred 756 bp downstream of the aroA start codon. Complementation of the aroA mutant with a plasmid carrying aroA recovered the wild-type phenotype. The mutant exhibited a 50% lethal dose (1 x 10(6) CFU/mouse) higher than that of the parental strain (4.3 x 10(4) CFU/mouse). The aroA mutant showed decreased ability to persist in the lungs, spleens, and mammary glands of mice. Intramammary immunization with the aroA mutant stimulated both Th1 and Th2 responses in the mammary gland, as ascertained by reverse transcription-PCR, and induced significant protection from challenge with either the parental wild-type or a heterologous strain isolated from a cow with mastitis.
Figures


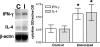
References
-
- Bentley, R. 1990. The shikimate pathway: a metabolic tree with many branches. Crit. Rev. Biochem. Mol. Biol. 25:307-384. - PubMed
-
- Brouillette, E., and F. Malouin. 2005. The pathogenesis and control of Staphylococcus aureus-induced mastitis: study models in the mouse. Microbes Infect. 7:560-568. - PubMed
-
- Brown, F., G. Dougan, E. M. Hoey, S. J. Martin, B. K. Rima, and A. Trudgett. 1993. Vaccine design. John Wiley & Sons, Chichester, England.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

