A cyanobacterial LPS antagonist prevents endotoxin shock and blocks sustained TLR4 stimulation required for cytokine expression
- PMID: 16717116
- PMCID: PMC2118317
- DOI: 10.1084/jem.20060136
A cyanobacterial LPS antagonist prevents endotoxin shock and blocks sustained TLR4 stimulation required for cytokine expression
Abstract
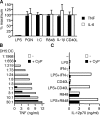
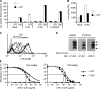
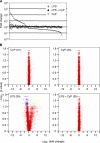
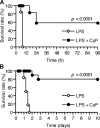
Toll-like receptors (TLRs) function as primary sensors that elicit coordinated innate immune defenses through recognition of microbial products and induction of immune and proinflammatory genes. Here we report the identification and biological characterization of a lipopolysaccharide (LPS)-like molecule extracted from the cyanobacterium Oscillatoria Planktothrix FP1 (cyanobacterial product [CyP]) that is not stimulatory per se but acts as a potent and selective antagonist of bacterial LPS. CyP binds to MD-2 and efficiently competes with LPS for binding to the TLR4-MD-2 receptor complex. The addition of CyP together with LPS completely inhibited both MyD88- and TRIF-dependent pathways and suppressed the whole LPS-induced gene transcription program in human dendritic cells (DCs). CyP protected mice from endotoxin shock in spite of a lower capacity to inhibit LPS stimulation of mouse DCs. Interestingly, the delayed addition of CyP to DCs responding to LPS strongly inhibited signaling and cytokine production by immediate down-regulation of inflammatory cytokine mRNAs while not affecting other aspects of DC maturation, such as expression of major histocompatibility complex molecules, costimulatory molecules, and CCR7. Collectively, these results indicate that CyP is a potent competitive inhibitor of LPS in vitro and in vivo and reveal the requirement of sustained TLR4 stimulation for induction of cytokine genes in human DCs.
Figures
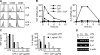


References
-
- Iwasaki, A., and R. Medzhitov. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5:987–995. - PubMed
-
- Lanzavecchia, A., and F. Sallusto. 2001. Regulation of T cell immunity by dendritic cells. Cell. 106:263–266. - PubMed
-
- Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. - PubMed
-
- Reis e Sousa, C. 2001. Dendritic cells as sensors of infection. Immunity. 14:495–498. - PubMed
-
- Kapsenberg, M.L. 2003. Dendritic-cell control of pathogen-driven T-cell polarization. Nat. Rev. Immunol. 3:984–993. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

