An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate
- PMID: 16717191
- PMCID: PMC1482500
- DOI: 10.1073/pnas.0600942103
An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate
Abstract
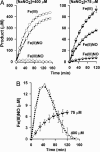
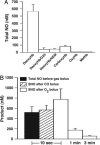
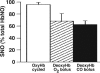
Red blood cells (RBCs) act as O(2)-responsive transducers of vasodilator and vasoconstrictor activity in lungs and tissues by regulating the availability of nitric oxide (NO). Vasodilation by RBCs is impaired in diseases characterized by hypoxemia. We have proposed that the extent to which RBCs constrict vs. dilate vessels is, at least partly, controlled by a partitioning between NO bound to heme iron and to Cysbeta93 thiol of hemoglobin (Hb). Hemes sequester NO, whereas thiols deploy NO bioactivity. In recent work, we have suggested that specific micropopulations of NO-liganded Hb could support the chemistry of S-nitrosohemoglobin (SNO-Hb) formation. Here, by using nitrite as the source of NO, we demonstrate that a (T state) micropopulation of a heme-NO species, with spectral and chemical properties of Fe(III)NO, acts as a precursor to SNO-Hb formation, accompanying the allosteric transition of Hb to the R state. We also show that at physiological concentrations of nitrite and deoxyHb, a S-nitrosothiol precursor is formed within seconds and produces SNO-Hb in high yield upon its prompt exposure to O(2) or CO. Deoxygenation/reoxygenation cycling of oxyHb in the presence of physiological amounts of nitrite also efficiently produces SNO-Hb. In contrast, high amounts of nitrite or delays in reoxygenation inhibit the production of SNO-Hb. Collectively, our data provide evidence for a physiological S-nitrosothiol synthase activity of tetrameric Hb that depends on NO-Hb micropopulations and suggest that dysfunction of this activity may contribute to the pathophysiology of cardiopulmonary and blood disorders.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

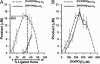
References
-
- Jia L., Bonaventura C., Bonaventura J., Stamler J. S. Nature. 1996;380:221–226. - PubMed
-
- Stamler J. S., Jia L., Eu J. P., McMahon T. J., Demchenko I. T., Bonaventura J., Gernert K., Piantadosi C. A. Science. 1997;276:2034–2037. - PubMed
-
- McMahon T. J., Moon R. E., Luschinger B. P., Carraway M. S., Stone A. E., Stolp B. W., Gow A. J., Pawloski J. R., Watke P., Singel D. J., et al. Nat. Med. 2002;8:711–717. - PubMed
-
- Dietrich H. H., Ellsworth M. L., Sprague R. S., Dacey R. G., Jr Am. J. Physiol. 2000;278:H1294–H1298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

