Frontotemporal dementia: clinicopathological correlations
- PMID: 16718704
- PMCID: PMC2629792
- DOI: 10.1002/ana.20873
Frontotemporal dementia: clinicopathological correlations
Abstract
Objective: Frontotemporal lobar degeneration (FTLD) is characterized by impairments in social, behavioral, and/or language function, but postmortem studies indicate that multiple neuropathological entities lead to FTLD. This study assessed whether specific clinical features predict the underlying pathology.
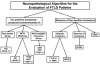
Methods: A clinicopathological correlation was performed on 90 consecutive patients with a pathological diagnosis of frontotemporal dementia and was compared with an additional 24 cases accrued during the same time period with a clinical diagnosis of FTLD, but with pathology not typically associated with frontotemporal dementia.
Results: Postmortem examination showed multiple pathologies including tauopathies (46%), FTLD with ubiquitin-positive inclusions (29%), and Alzheimer's disease (17%). The pathological groups manifested some distinct demographic, clinical, and neuropsychological features, although these attributes showed only a statistical association with the underlying pathology. FTLD with ubiquitin-positive inclusions was more likely to present with both social and language dysfunction, and motor neuron disease was more likely to emerge in these patients. Tauopathies were more commonly associated with an extrapyramidal disorder. Alzheimer's disease was associated with relatively greater deficits in memory and executive function.
Interpretation: Clinical and neuropsychological features contribute to delineating the spectrum of pathology underlying a patient diagnosed with FTLD, but biomarkers are needed that, together with the clinical phenotype, can predict the underlying neuropathology.
Ann Neurol 2006;59:952-962
Figures



References
-
- Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. - PubMed
-
- Grossman M. Frontotemporal dementia: a review. J Int Neuropsychol Soc. 2002;8:566–583. - PubMed
-
- McKhann GM, Albert MS, Grossman M, et al. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch Neurol. 2001;58:1803–1809. - PubMed
-
- Grossman M, Ash S. Primary progressive aphasia: a review. Neurocase. 2004;10:3–18. - PubMed
-
- Hodges JR, Patterson K, Oxbury S, et al. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115(pt 6):1783–1806. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS044266/NS/NINDS NIH HHS/United States
- P30 AG010124/AG/NIA NIH HHS/United States
- P01 AG17586/AG/NIA NIH HHS/United States
- P01 AG009215/AG/NIA NIH HHS/United States
- R01 NS44266/NS/NINDS NIH HHS/United States
- K08 NS041408/NS/NINDS NIH HHS/United States
- K08 AG20073/AG/NIA NIH HHS/United States
- P01 AG019724/AG/NIA NIH HHS/United States
- R01 AG022538/AG/NIA NIH HHS/United States
- L30 AG024692/AG/NIA NIH HHS/United States
- P30 AG10124/AG/NIA NIH HHS/United States
- R01 AG15116/AG/NIA NIH HHS/United States
- R01 AG015116/AG/NIA NIH HHS/United States
- P01 AG017586/AG/NIA NIH HHS/United States
- K08 NS41408/NS/NINDS NIH HHS/United States
- K08 AG020073/AG/NIA NIH HHS/United States
- P01 AG19724/AG/NIA NIH HHS/United States
- P01 AG09215/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

