Current and future applications of magnetic resonance imaging and spectroscopy of the brain in hepatic encephalopathy
- PMID: 16718775
- PMCID: PMC4124369
- DOI: 10.3748/wjg.v12.i19.2969
Current and future applications of magnetic resonance imaging and spectroscopy of the brain in hepatic encephalopathy
Abstract
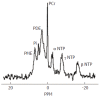
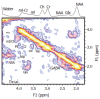
Hepatic encephalopathy (HE) is a common neuro-psychiatric abnormality, which complicates the course of patients with liver disease and results from hepatocellular failure and/or portosystemic shunting. The manifestations of HE are widely variable and involve a spectrum from mild subclinical disturbance to deep coma. Research interest has focused on the role of circulating gut-derived toxins, particularly ammonia, the development of brain swelling and changes in cerebral neurotransmitter systems that lead to global CNS depression and disordered function. Until recently the direct investigation of cerebral function has been difficult in man. However, new magnetic resonance imaging (MRI) techniques provide a non-invasive means of assessment of changes in brain volume (coregistered MRI) and impaired brain function (fMRI), while proton magnetic resonance spectroscopy (1H MRS) detects changes in brain biochemistry, including direct measurement of cerebral osmolytes, such as myoinositol, glutamate and glutamine which govern processes intrinsic to cellular homeostasis, including the accumulation of intracellular water. The concentrations of these intracellular osmolytes alter with hyperammonaemia. MRS-detected metabolite abnormalities correlate with the severity of neuropsychiatric impairment and since MR spectra return towards normal after treatment, the technique may be of use in objective patient monitoring and in assessing the effectiveness of various treatment regimens.
Figures

References
-
- Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716–721. - PubMed
-
- Conn HO. Diuresis of ascites: fraught with or free from hazard. Gastroenterology. 1977;73:619–621. - PubMed
-
- Butterworth RF, Giguère JF, Michaud J, Lavoie J, Layrargues GP. Ammonia: key factor in the pathogenesis of hepatic encephalopathy. Neurochem Pathol. 1987;6:1–12. - PubMed
-
- Schenker S, Brady CE. Pathogenesis of hepatic encephalopathy. In: Conn , HO , Bircher , J , editors. Hepatic Encephalopathy: Syndromes and Therapies. Bloomington: Medi-Ed Press; 1994. pp. 43–61.
-
- Häussinger D, Kircheis G, Fischer R, Schliess F, vom Dahl S. Hepatic encephalopathy in chronic liver disease: a clinical manifestation of astrocyte swelling and low-grade cerebral edema. J Hepatol. 2000;32:1035–1038. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

