Phase II trial of oral S-1 combined with gemcitabine in metastatic pancreatic cancer
- PMID: 16721372
- PMCID: PMC2361295
- DOI: 10.1038/sj.bjc.6603168
Phase II trial of oral S-1 combined with gemcitabine in metastatic pancreatic cancer
Abstract
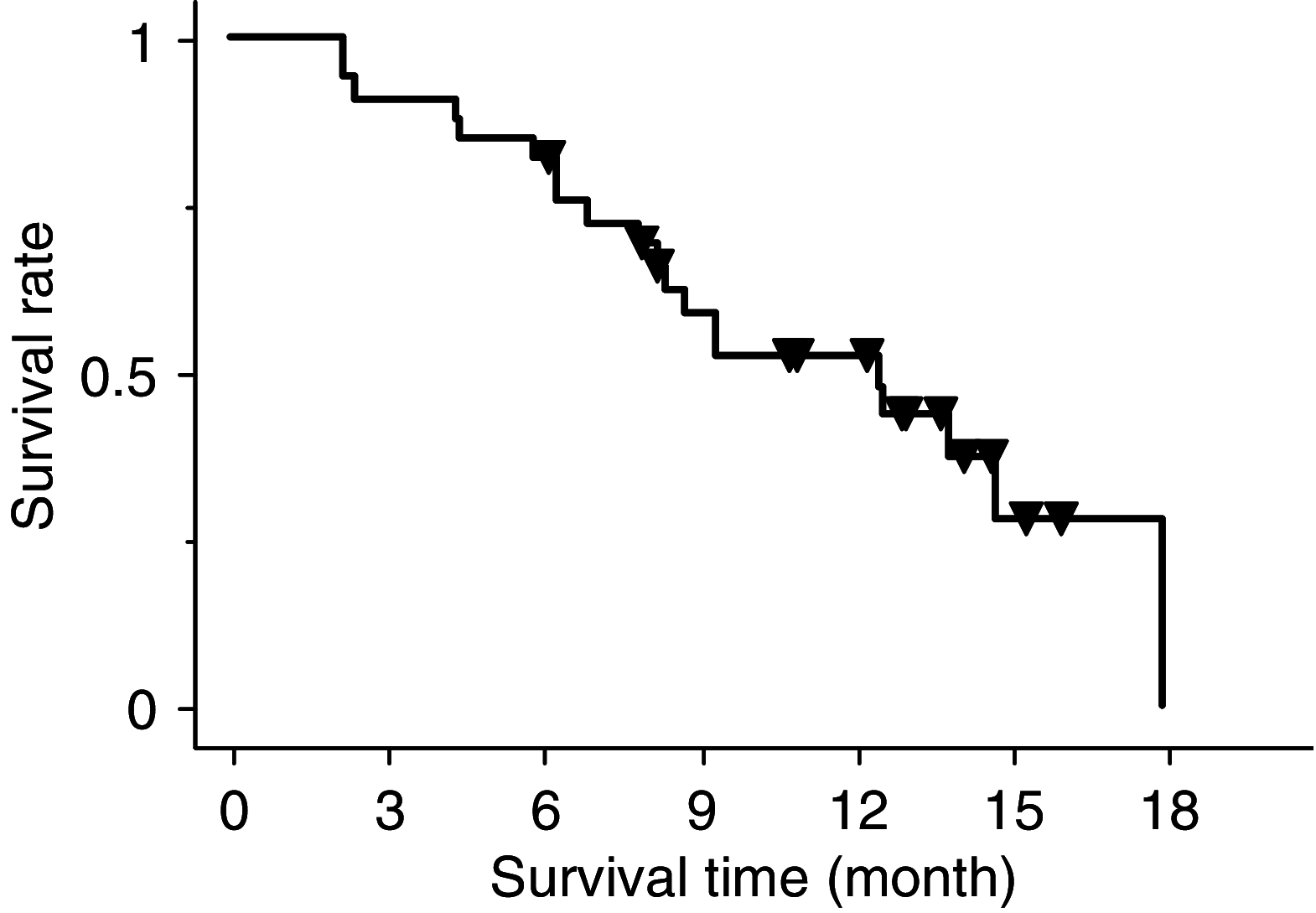
We conducted a phase II trial of gemcitabine with S-1, oral fluorouracil (5-FU) prodrug tegafur combined with two modulators, 5-chloro-2, 4-dihydroxypyridine and potassium oxonate, to evaluate the activity and toxicity of such a combination in metastatic pancreatic cancer (MPC) patients. Patients who had pathologically proven pancreatic cancer with metastatic lesions were eligible candidates for entry into the study. S-1 was given orally (30 mg m(-2)) b.i.d. for 14 consecutive days and gemcitabine (1000 mg m(-2)) was given on days 8 and 15. The cycle was repeated every 21 days. We enrolled 33 MPC patients. The median number of cycles was eight (range 1-20). Grade 3-4 toxicities were leucopenia (33%), neutropenia (55%), anaemia (9%), thrombocytopenia (15%), anorexia (6%), fever (9%), and interstitial pneumonia (6%). Objective responses were obtained in 16 patients (one complete response and 15 partial responses; response rate, 48%; 95% confidence interval (CI), 33-65). Median survival and 1-year survival rate were 12.5 months (95% CI, 5.9-19.1) and 54% (95% CI, 36-72), respectively. Combination chemotherapy with GEM and S-1 was well tolerated and yielded a significantly high response rate.
Figures
References
-
- Bruckner H, Zhou G, Haenel P (1998) Ex vivo ATP tumor testing of gemcitabine for combination chemotherapy and biochemical modulation. Proc Am Assoc Cancer Res 89: 310 (abstract 2116)
-
- Burris III HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15: 2403–2413 - PubMed
-
- Chollet P, Schoffski P, Weigang-Kohler K, Schellens JH, Cure H, Pavlidis N, Grunwald V, De Boer R, Wanders J, Fumoleau P (2003) Phase II trial with S-1 in chemotherapy-naive patients with gastric cancer. A trial performed by the EORTC Early Clinical Studies Group (ECSG). Eur J Cancer 39: 1264–1270 - PubMed
-
- Evans D, Abbruzzese J, Rich T (1997) Cancer of the pancreas. In Cancer: Principles and Practice of Oncology, De Vita VJ, Hellman S, Rosenberg S (eds) pp 1054–1077. Philadelphia, PA: Lippincott
-
- Feliu J, Lopez Alvarez MP, Jaraiz MA, Constenla M, Vicent JM, Belon J, Lopez Gomez L, de Castro J, Dorta J, Gonzalez Baron M (2000) Phase II trial of gemcitabine and UFT modulated by leucovorin in patients with advanced pancreatic carcinoma. The ONCOPAZ Cooperative Group. Cancer 89: 1706–1713 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials