Persistently elevated T cell interferon-gamma responses after treatment for latent tuberculosis infection among health care workers in India: a preliminary report
- PMID: 16722616
- PMCID: PMC1481589
- DOI: 10.1186/1745-6673-1-7
Persistently elevated T cell interferon-gamma responses after treatment for latent tuberculosis infection among health care workers in India: a preliminary report
Abstract
Background: T cell-based interferon-gamma (IFN-gamma) release assays (IGRAs) are novel tests for latent tuberculosis infection (LTBI). It has been suggested that T cell responses may be correlated with bacterial burden and, therefore, IGRAs may have a role in monitoring treatment response. We investigated IFN-gamma responses to specific TB antigens among Indian health care workers (HCWs) before, and after LTBI preventive therapy.
Methods: In 2004, we established a cohort of HCWs who underwent tuberculin skin testing (TST) and a whole-blood IGRA (QuantiFERON-TB-Gold In-Tube [QFT-G], Cellestis Ltd, Victoria, Australia) at a rural hospital in India. HCWs positive by either test were offered 6 months of isoniazid (INH) preventive therapy. Among the HCWs who underwent therapy, we prospectively followed-up 10 nursing students who were positive by both tests at baseline. The QFT-G assay was repeated 4 and 10 months after INH treatment completion (i.e. approximately 12 months and 18 months after the initial testing). IFN-gamma responses to ESAT-6, CFP-10 and TB7.7 peptides were measured using ELISA, and IFN-gamma >/=0.35 IU/mL was used to define a positive QFT-G test result.
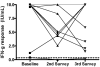
Results: All participants (N = 10) reported direct contact with smear-positive TB patients at baseline, during and after LTBI treatment. All participants except one started treatment with high baseline IFN-gamma responses (median 10.0 IU/mL). The second QFT-G was positive in 9 of 10 participants, but IFN-gamma responses had declined (median 5.0 IU/mL); however, this difference was not significant (P = 0.10). The third QFT-G assay continued to be positive in 9 of 10 participants, with persistently elevated IFN-gamma responses (median 7.9 IU/mL; P = 0.32 for difference against baseline average).
Conclusion: In an environment with ongoing, intensive nosocomial exposure, HCWs had strong IFN-gamma responses at baseline, and continued to have persistently elevated responses, despite LTBI treatment. It is plausible that persistence of infection and/or re-infection might account for this phenomenon. Our preliminary findings need confirmation in larger studies in high transmission settings. Specifically, research is needed to study T cell kinetics during LTBI treatment, and determine the effect of recurrent exposures on host cellular immune responses.
Figures
References
-
- World Health Organization . Global tuberculosis control. Surveillance, planning, financing. WHO Report 2005. Geneva , World Health Organization; 2005. pp. 1–247.
-
- Menzies RI. Tuberculin skin testing. In: Reichman LB, Hershfield ES, editor. Tuberculosis: a comprehensive international approach. New York , Marcel Dekker; 2000. pp. 279–322.
-
- Huebner RE, Schein MF, Bass JBJ. The tuberculin skin test. Clin Infect Dis. 1993;17:968–975. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

